Minimally invasive molecular delivery into the brain using optical modulation of vascular permeability
- PMID: 21576460
- PMCID: PMC3107279
- DOI: 10.1073/pnas.1018790108
Minimally invasive molecular delivery into the brain using optical modulation of vascular permeability
Abstract
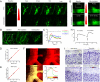
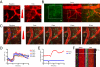
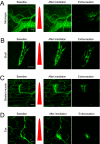
Systemic delivery of bioactive molecules in the CNS is hampered by the blood-brain barrier, which has bottlenecked noninvasive physiological study of the brain and the development of CNS drugs. Here we report that irradiation with an ultrashort pulsed laser to the blood vessel wall induces transient leakage of blood plasma without compromising vascular integrity. By combining this method with a systemic injection, we delivered target molecules in various tissues, including the brain cortex. This tool allows minimally invasive local delivery of chemical probes, nanoparticles, and viral vectors into the brain cortex. Furthermore, we demonstrated astrocyte-mediated vasodilation in vivo without opening the skull, using this method to load a calcium indicator in conjunction with label-free photoactivation of astrocytes.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Brightman MW. Morphology of blood–brain interfaces. Exp Eye Res. 1977;25(Suppl):1–25. - PubMed
-
- Pardridge WM. Blood–brain barrier delivery. Drug Discov Today. 2007;12:54–61. - PubMed
-
- Xu HT, Pan F, Yang G, Gan WB. Choice of cranial window type for in vivo imaging affects dendritic spine turnover in the cortex. Nat Neurosci. 2007;10:549–551. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

