Repair of injured proximal tubule does not involve specialized progenitors
- PMID: 21576461
- PMCID: PMC3107336
- DOI: 10.1073/pnas.1100629108
Repair of injured proximal tubule does not involve specialized progenitors
Abstract
Recently we have established that the kidney tubular epithelium is repaired by surviving epithelial cells. It is not known, however, whether a population of intratubular adult progenitor cells are responsible for this epithelial repair after acute kidney injury. In this study, we used an unbiased DNA analog-based approach that does not rely on candidate markers to track multiple rounds of cell division in vivo. In the proximal tubule, robust thymidine analog incorporation was observed postinjury. Cell division was stochastic and enriched among cells that were injured and dedifferentiated. There was no evidence for the presence of a population of specialized progenitors that repeatedly divide in response to injury. Instead, these results indicate that after injury, new epithelial cells arise from self-duplication of surviving cells, most of which are injured. Because the renal papilla contains DNA label-retaining cells and has been proposed as a stem cell niche, we examined the proliferative behavior of these putative progenitors after ischemia-reperfusion injury. Although label-retaining cells in the renal papilla diminished with time after ischemia-reperfusion injury, they neither proliferated nor migrated to the outer medulla or cortex. Thus, nonlethally injured cells repopulate the kidney epithelium after injury in the absence of any specialized progenitor cell population.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
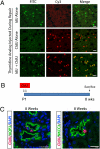
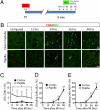
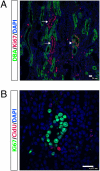

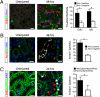
References
-
- Messier B, Leblond CP. Cell proliferation and migration as revealed by radioautography after injection of thymidine-H3 into male rats and mice. Am J Anat. 1960;106:247–285. - PubMed
-
- Venkatachalam MA, Bernard DB, Donohoe JF, Levinsky NG. Ischemic damage and repair in the rat proximal tubule: Differences among the S1, S2, and S3 segments. Kidney Int. 1978;14(1):31–49. - PubMed
-
- Witzgall R, Brown D, Schwarz C, Bonventre JV. Localization of proliferating cell nuclear antigen, vimentin, c-Fos, and clusterin in the postischemic kidney. Evidence for a heterogenous genetic response among nephron segments, and a large pool of mitotically active and dedifferentiated cells. J Clin Invest. 1994;93:2175–2188. - PMC - PubMed
-
- Little MH, Bertram JF. Is there such a thing as a renal stem cell? J Am Soc Nephrol. 2009;20:2112–2117. - PubMed
-
- Elger M, et al. Nephrogenesis is induced by partial nephrectomy in the elasmobranch Leucoraja erinacea. J Am Soc Nephrol. 2003;14:1506–1518. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

