Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer
- PMID: 21576639
- PMCID: PMC3422534
- DOI: 10.1200/JCO.2010.32.0630
Clinical end points and response criteria in mycosis fungoides and Sézary syndrome: a consensus statement of the International Society for Cutaneous Lymphomas, the United States Cutaneous Lymphoma Consortium, and the Cutaneous Lymphoma Task Force of the European Organisation for Research and Treatment of Cancer
Abstract
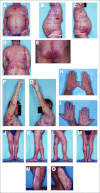
Mycosis fungoides (MF) and Sézary syndrome (SS), the major forms of cutaneous T-cell lymphoma, have unique characteristics that distinguish them from other types of non-Hodgkin's lymphomas. Clinical trials in MF/SS have suffered from a lack of standardization in evaluation, staging, assessment, end points, and response criteria. Recently defined criteria for the diagnosis of early MF, guidelines for initial evaluation, and revised staging and classification criteria for MF and SS now offer the potential for uniform staging of patients enrolled in clinical trials for MF/SS. This article presents consensus recommendations for the general conduct of clinical trials of patients with MF/SS as well as methods for standardized assessment of potential disease manifestations in skin, lymph nodes, blood, and visceral organs, and definition of end points and response criteria. These guidelines should facilitate collaboration among investigators and collation of data from sponsor-generated or investigator-initiated clinical trials involving patients with MF or SS.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures
References
-
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: A proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Blood. 2007;110:1713–1722. - PubMed
-
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768–3785. - PubMed
-
- Lutzner M, Edelson R, Schein P, et al. Cutaneous T-cell lymphomas: The Sézary syndrome, mycosis fungoides, and related disorders. Ann Intern Med. 1975;83:534–552. - PubMed
-
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: Clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857–866. - PubMed
-
- Bunn PA, Jr, Lamberg SI. Report of the Committee on Staging and Classification of Cutaneous T-Cell Lymphomas. Cancer Treat Rep. 1979;63:725–728. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

