Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice
- PMID: 21576823
- PMCID: PMC3104738
- DOI: 10.1172/JCI43157
Repeated TLR9 stimulation results in macrophage activation syndrome-like disease in mice
Abstract
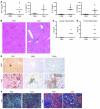
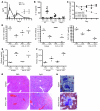
Hemophagocytic lymphohistiocytosis (HLH) and macrophage activation syndrome (MAS) are 2 similar diseases characterized by a cytokine storm, overwhelming inflammation, multiorgan dysfunction, and death. Animal models of HLH suggest that disease is driven by IFN-γ produced by CD8⁺ lymphocytes stimulated by persistent antigen exposure. In these models and patients with "primary" HLH, the antigen persists due to genetic defects, resulting in ineffective cytotoxic responses by CD8⁺ T cells and poor pathogen clearance. However, infectious triggers are often not identified in patients with MAS, and some patients with HLH or MAS lack defects in cytotoxic T cell killing. Herein, we show that repeated stimulation of TLR9 produced an HLH/MAS-like syndrome on a normal genetic background, without exogenous antigen. Like previous HLH models, TLR9-induced MAS was IFN-γ dependent; however, unlike other models, disease did not require lymphocytes. We further showed that IL-10 played a protective role in this model and that blocking IL-10 signaling led to the development of hemophagocytosis. IL-10 may therefore be an important target for the development of effective therapeutics for MAS. Our data provide insight into MAS-like syndromes in patients with inflammatory diseases in which there is chronic innate immune activation but no genetic defects in cytotoxic cell function.
Figures


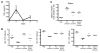



References
-
- Castillo L, Carcillo J. Secondary hemophagocytic lymphohistiocytosis and severe sepsis/ systemic inflammatory response syndrome/multiorgan dysfunction syndrome/macrophage activation syndrome share common intermediate phenotypes on a spectrum of inflammation. Pediatr Crit Care Med. 2009;10(3):387–392. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

