The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice
- PMID: 21576824
- PMCID: PMC3104778
- DOI: 10.1172/JCI57367
The AAV9 receptor and its modification to improve in vivo lung gene transfer in mice
Abstract
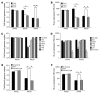
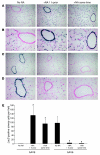
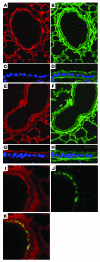
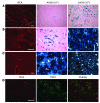
Vectors based on adeno-associated virus (AAV) serotype 9 are candidates for in vivo gene delivery to many organs, but the receptor(s) mediating these tropisms have yet to be defined. We evaluated AAV9 uptake by glycans with terminal sialic acids (SAs), a common mode of cellular entry for viruses. We found, however, that AAV9 binding increased when terminal SA was enzymatically removed, suggesting that galactose, which is the most commonly observed penultimate monosaccharide to SA, may mediate AAV9 transduction. This was confirmed in mutant CHO Pro-5 cells deficient in the enzymes involved in glycoprotein biogenesis, as well as lectin interference studies. Binding of AAV9 to glycans with terminal galactose was demonstrated via glycan binding assays. Co-instillation of AAV9 vector with neuraminidase into mouse lung resulted in exposure of terminal galactose on the apical surface of conducting airway epithelial cells, as shown by lectin binding and increased transduction of these cells, demonstrating the possible utility of this vector in lung-directed gene transfer. Increasing the abundance of the receptor on target cells and improving vector efficacy may improve delivery of AAV vectors to their therapeutic targets.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

