2,4,6,8-Tetra-kis(2-methoxy-phen-yl)-3,7-diaza-bicyclo-[3.3.1]nonan-9-one diethyl ether hemisolvate
- PMID: 21577937
- PMCID: PMC2970300
- DOI: 10.1107/S1600536809036733
2,4,6,8-Tetra-kis(2-methoxy-phen-yl)-3,7-diaza-bicyclo-[3.3.1]nonan-9-one diethyl ether hemisolvate
Abstract
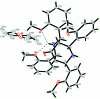
In the title compound, C(35)H(36)N(2)O(5)·0.5C(4)H(10)O, the asymmetric unit contains one bicyclo-[3.3.1]nonane mol-ecule and a half-occupancy diethyl ether solvent with the O atom lying on a crystallographic inversion center. Two intra-molecular N-H⋯O hydrogen bonds generate S(6) ring motifs. The bicyclo-[3.3.1]nonane ring system adopts a chair-boat conformation. In the crystal structure, the mol-ecules are linked by weak inter-molecular C-H⋯N hydro-gen bonds into chains along the b axis; additional stabilization is provide by C-H⋯π inter-actions.
Figures

References
-
- Arias-Perez, M. S., Alejo, A. & Maroto, A. (1997). Tetrahedron, 53, 13099–13110.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc.97, 1354–1358.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
