Tetra-kis(2,6-diamino-pyridinium) diphthalate 2,6-diamino-pyridine
- PMID: 21578508
- PMCID: PMC2971263
- DOI: 10.1107/S1600536809044468
Tetra-kis(2,6-diamino-pyridinium) diphthalate 2,6-diamino-pyridine
Abstract
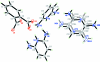
In the title compound, 4C(5)H(8)N(3) (+)·2C(8)H(4)O(4) (2-)·C(5)H(7)N(3), the asymmetric unit consists of two protonated diamino-pyridine cations, one phthalate anion and one half of a diamino-pyridine mol-ecule, which has twofold rotation symmetry and is disordered over two positions with a site-occupancy ratio of 0.534 (3):0.466 (3). In the disordered structure, both pyridine rings are essentially planar, with maximum deviations of 0.011 (2) and 0.006 (2) Å, and these two rings are inclined to one another at a dihedral angle of 79.86 (10)°. In the crystal structure, inter-molecular N-H⋯O and C-H⋯O hydrogen bonds link the ions and mol-ecules into a three-dimensional network. The structure is further stabilized by C-H⋯π inter-actions.
Figures

References
-
- Abu Zuhri, A. Z. & Cox, J. A. (1989). Mikrochim. Acta, 11, 277–283.
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Brike, G., Hirsch, M. & Franz, V. (2002). US Patent No. 636 838 9B1.
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Büyükgüngör, O. & Odabąsoğlu, M. (2006). Acta Cryst. E62, o3816–o3818.
LinkOut - more resources
Full Text Sources
