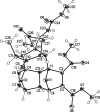
Dimethyl 11,13-dimethyl-16-[1,2-bis-(methoxy-carbon-yl)ethen-yl]-12-oxo-16,17-dioxa-18-aza-hexa-cyclo-[7.5.1.1.1.1.0]octa-deca-2,7-diene-2,3-dicarboxyl-ate
- PMID: 21578945
- PMCID: PMC2972130
- DOI: 10.1107/S1600536809050600
Dimethyl 11,13-dimethyl-16-[1,2-bis-(methoxy-carbon-yl)ethen-yl]-12-oxo-16,17-dioxa-18-aza-hexa-cyclo-[7.5.1.1.1.1.0]octa-deca-2,7-diene-2,3-dicarboxyl-ate
Abstract
The title compound, C(27)H(29)NO(11), is a product of the tandem 'domino' Diels-Alder reaction. The mol-ecule comprises a fused hexa-cyclic system containing four five-membered rings (two dihydro-furan and two tetra-hydro-furan) in the usual envelope conformations and two six-membered rings (tetra-hydro-pyridinone and piperidine) adopting slightly flattened boat and chair conformations, respectively. The dispositions of the carboxyl-ate substituents relative to each other are determined by both steric reasons and inter-molecular C-H⋯O hydrogen bonding and attractive anti-parallel C=O⋯C=O inter-actions [C⋯O = 2.995 (2) Å].
Figures
References
-
- Bruker (1998). SMART and SAINT-Plus. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Domingo, L. R., Picher, M. T. & Andres, J. (2000). J. Org. Chem. 65, 3473–3477. - PubMed
-
- Lautens, M. & Fillion, E. (1996). J. Org. Chem. 61, 7994–7995. - PubMed
-
- Lautens, M. & Fillion, E. (1997). J. Org. Chem. 62, 4418–4427. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous