Dicobalt copper bis-[orthophosphate(V)] monohydrate, Co(2.39)Cu(0.61)(PO(4))(2)·H(2)O
- PMID: 21578990
- PMCID: PMC2979060
- DOI: 10.1107/S1600536810015382
Dicobalt copper bis-[orthophosphate(V)] monohydrate, Co(2.39)Cu(0.61)(PO(4))(2)·H(2)O
Abstract
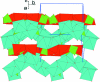
In an attempt to hydro-thermally synthesize a phase with composition Co(2)Cu(PO(4))(2)·H(2)O, we obtained the title compound, Co(2.39)Cu(0.61)(PO(4))(2)·H(2)O instead. Chemical analysis confirmed the presence of copper in the crystal. The crystal structure of the title compound can be described as a three- dimensional network constructed from the stacking of two types of layers extending parallel to (010). These layers are made up from more or less deformed polyhedra: CoO(6) octa-hedra, (Cu/Co)O(5) square pyramids and PO(4) tetra-hedra. The first layer is formed by pairs of edge-sharing (Cu/Co)O(5) square pyramids linked via a common edge of each end of the (Cu/Co)(2)O(8) dimer to PO(4) tetra-hedra. The second layer is undulating and is built up from edge-sharing CoO(6) octa-hedra. The linkage between the two layers is accomplished by PO(4) tetra-hedra. The presence of water mol-ecules in the CoO(4)(H(2)O)(2) octa-hedron also contributes to the cohesion of the layers through O-H⋯O hydrogen bonding.
Figures



References
-
- Anderson, J., Kostiner, E. & Ruszala, F. A. (1976). Inorg. Chem.15 2744–2748.
-
- Brandenburg, K. (2006). DIAMOND Crystal Impact GbR, Bonn, Germany.
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Clearfield, A. (1988). Chem. Rev.88, 125–148,
-
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
LinkOut - more resources
Full Text Sources
Miscellaneous
