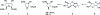2-Acetamido-N-benzyl-1,4-imino-1,2,4-tride-oxy-l-xylitol (N-benzyl-l-XYLNAc)
- PMID: 21579195
- PMCID: PMC2979175
- DOI: 10.1107/S1600536810014145
2-Acetamido-N-benzyl-1,4-imino-1,2,4-tride-oxy-l-xylitol (N-benzyl-l-XYLNAc)
Abstract
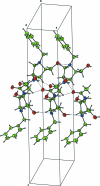
X-ray crystallography defines the relative configuration at the three-stereogenic centres in the title compound N-benzyl-l-XYLNAc, C(14)H(20)N(2)O(3). The five-membered pyrrolidine ring adopts an envelope conformation with the N atom lying out of the plane of the other four atoms. In the crystal structure, inter-molecular O-H⋯O, N-H⋯O and O-H⋯N hydrogen bonds link the mol-ecules into chains along [100]. The carbonyl group O atom acts as an acceptor for a bifurcated hydrogen bond. The absolute configuration is determined by the use of l-glucuronolactone as the starting material for the synthesis.
Figures
References
-
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
-
- Asano, N., Nash, R. J., Molyneux, R. J. & Fleet, G. W. J. (2000). Tetrahedron Asymmetry, 11, 1645–1680.
-
- Best, D., Chairatana, P., Glawar, A. F. G., Crabtree, E., Butters, T. D., Wilson, F. X., Yu, C.-Y., Wang, W.-B., Jia, Y.-M., Adachi, I., Kato, A. & Fleet, G. W. J. (2010). Tetrahedron Lett.51, 2222–2224.
-
- Best, D., Wang, C., Weymouth-Wilson, A. C., Clarkson, R. A., Wilson, F. X., Nash, R. J., Miyauchi, S., Kato, A. & Fleet, G. W. J. (2010). Tetrahedron Asymmetry, 21, 311–319.
-
- Betteridge, P. W., Carruthers, J. R., Cooper, R. I., Prout, K. & Watkin, D. J. (2003). J. Appl. Cryst.36, 1487.
Grants and funding
LinkOut - more resources
Full Text Sources