5-Methyl-3,6,7,8a-tetra-hydro-2H-diimidazo[1,2-c:1',2'-e]pyrido[1,2-a][1,3,5]triazin-5-ium iodide
- PMID: 21579550
- PMCID: PMC2979596
- DOI: 10.1107/S1600536810019495
5-Methyl-3,6,7,8a-tetra-hydro-2H-diimidazo[1,2-c:1',2'-e]pyrido[1,2-a][1,3,5]triazin-5-ium iodide
Abstract
The structure of the title compound, C(12)H(16)N(5) (+)·I(-), shows that the methyl-ation reaction with CH(3)I occurred at the imine N atom at position 5 of the 3,6,7,8a-tetra-hydro-2H-diimidazo[1,2-c:1',2'-e]pyrido[1,2-a][1,3,5]triazine system. In the cation, the sp(3)-hybridized C atom belonging to the fused dihydro-pyrine and dihydro-1,3,5-triazine rings deviates by 0.514 (3) Å from the best plane defined by the remaining cationic non-H atoms. The fused dihydro-pyridine and dihydro-1,3,5-triazine rings are each in a half-chair conformation with the sp(3)-hybridized C atom as a flap. The iodide anion is 3.573 (2) Å from the methyl-ated N atom and exhibits five short C-H⋯I(-) contacts with distances less than 3.16 Å. The structure has been determined from a non-merohedral twin with twin law [-1 0 0 0 - 1 0 0.115 0 1], minor domain = 0.1559 (12).
Figures


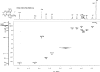
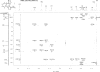
References
-
- Cooper, R. I., Gould, R. O., Parsons, S. & Watkin, D. J. (2002). J. Appl. Cryst.35, 168–174.
-
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
-
- Farrugia, L. J. (1999). J. Appl. Cryst.32, 837–838.
-
- Oxford Diffraction (2009). CrysAlis PRO Oxford Diffraction Ltd, Yarnton, England.
-
- Sączewski, F. & Foks, H. (1981). Synthesis, pp. 151–152.
LinkOut - more resources
Full Text Sources
