2-Propynyl 2-hydroxy-benzoate
- PMID: 21580108
- PMCID: PMC2980208
- DOI: 10.1107/S160053680905421X
2-Propynyl 2-hydroxy-benzoate
Abstract
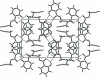
The title compound, C(10)H(8)O(3), has been synthesized as part of our investigations into the generation of new anti-bacterial agents and serves as a building block for the synthesis of compound libraries. The compound crystallizes with two independent mol-ecules in the asymmetric unit. The transoid propynyl ester groups are coplanar with the 2-hydroxy-benzoate group with maximum deviations of -0.3507 (3) and 0.1591 (3) Å for the terminal carbons, with intra-molecular O-H⋯O hydrogen bonding providing rigidity to the structure and ensuring that the reactivity of the alkyne is not compromised by steric factors. The propynyl group forms inter-molecular C-H⋯O inter-actions with the phenolic O atom. Supra-molecular chains along the b axis are found for both mol-ecules with links by weak O-H⋯O inter-molecular inter-actions in the first independent mol-ecule and C-H⋯O inter-actions in the second.
Figures
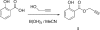

References
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
-
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
-
- Houston, T. A., Levonis, S. M. & Kiefel, M. J. (2007). Aust. J. Chem.60, 811–815.
-
- Houston, T. A., Wilkinson, B. L. & Blanchfield, J. T. (2004). Org. Lett.6, 678–681. - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
