Hexakis(tetra-aqua-sodium) deca-vanadate(V) dihydrate
- PMID: 21580463
- PMCID: PMC2983821
- DOI: 10.1107/S1600536810010251
Hexakis(tetra-aqua-sodium) deca-vanadate(V) dihydrate
Abstract
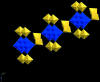
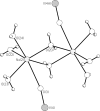
The title compound, {[Na(H(2)O)(4)](6)[V(10)O(28)]·2H(2)O}(n), crystallized from a H(2)O/THF/CH(3)CN solution (pH ca 6) containing equimolar amounts of NaVO(3) and N-(2-hydroxy-benz-yl)-N-(2-picol-yl)glycine. In the crystal structure, the deca-vanadate [V(10)O(28)](6-) anion ( symmetry) is coordinated, via four terminal oxide ligands of V centres, to two dinuclear [{Na(H(2)O)(3)}(2)(μ-H(2)O)(2)](2+) units. Inter-connection of these aquasodium-ion-sandwiched deca-vanadates to chains parallel to [001] is effected by μ-[{Na(H(2)O)(3)}(2)(μ-H(2)O)(2)](2+) units, bridging adjacent deca-vanadates via O=V. The structure is consolidated by an extensive network of O-H⋯O hydrogen bonds.
Figures

References
-
- Baruah, B., Roden, J. M., Sedgwick, M., Correra, N. M., Crans, D. C. & Levinger, N. E. (2006). J. Am. Chem. Soc.128, 12758–12765. - PubMed
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Crans, D. C., Mahroof-Tahir, M., Anderson, O. P. & Miller, M. M. (1994). Inorg. Chem.33, 5586–5590.
-
- Durif, A., Averbuch-Pouchot, M. T. & Guitel, J. C. (1980). Acta Cryst. B36, 680–682.
-
- Farahbakhsh, M., Koegeler, P., Schmidt, H. & Rehder, D. (1998). Inorg. Chem. Commun.1, 111–114.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
