trans-Diaqua-bis(2,2'-bipyridine-κN,N')ruthenium(II) bis-(trifluoro-methane-sulfonate)
- PMID: 21580841
- PMCID: PMC2959720
- DOI: 10.1107/S1600536808028195
trans-Diaqua-bis(2,2'-bipyridine-κN,N')ruthenium(II) bis-(trifluoro-methane-sulfonate)
Abstract
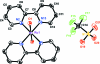
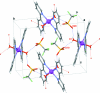
The title compound, trans-[Ru(bpy)(2)(H(2)O)(2)](CF(3)SO(3))(2) (bpy = 2,2'-bipyridine, C(10)H(8)N(2)), crystallized from the decomposition of an aged aqueous solution of a dimeric complex of cis-Ru(bpy)(2) in 0.1 M triflic acid. The Ru(II) ion is located on a crystallographic inversion center and exhibits a distorted octa-hedral coordination with equivalent ligands trans to each other. The Ru-O distance is 2.1053 (16) Å and the Ru-N distances are 2.0727 (17) and 2.0739 (17) Å. The bpy ligands are bent, due to inter-ligand steric inter-actions between H atoms of opposite pyridyl units across the Ru center. The crystal structure exhibits an extensive hydrogen-bonding network involving the water ligands and the trifluoromethane-sulfonate counter-ions within two-dimensional layers, although no close hydrogen-bond inter-actions exist between different layers.
Figures
References
-
- Bruker (2007). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Durham, B., Wilson, S. R., Hodgson, D. J. & Meyer, T. J. (1980). J. Am. Chem. Soc.102, 600–607.
-
- Jude, H., Rein, F. N., White, P. S., Dattelbaum, D. M. & Rocha, R. C. (2008). Inorg. Chem. 47, 7695–7702. - PubMed
-
- Klüfers, P. & Zangl, A. (2007). Acta Cryst. E63, m3088.
-
- Sheldrick, G. M. (2008). Acta Cryst. A64, 112–122. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
