4-Chloro-N-(4-chloro-phenyl-sulfon-yl)-N-(3-oxo-2,3-dihydro-1,2-benzisothia-zol-2-yl)benzene-sulfonamide
- PMID: 21582006
- PMCID: PMC2968287
- DOI: 10.1107/S1600536809003195
4-Chloro-N-(4-chloro-phenyl-sulfon-yl)-N-(3-oxo-2,3-dihydro-1,2-benzisothia-zol-2-yl)benzene-sulfonamide
Abstract
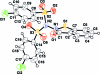
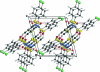
In the title compound, C(19)H(12)Cl(2)N(2)O(5)S(3), the benzene rings of the chloro-phenyl-sulfonyl groups form a dihedral angle of 35.85 (8)° and are inclined at angles of 23.51 (6) and 59.22 (6)° with respect to the essentially planar benzisothia-zole ring system [maximum deviation = 0.030 (2) Å]. The mol-ecular conformation is stabilized by an intra-molecular C-H⋯O hydrogen bond. In the crystal packing, mol-ecules are linked into chains parallel to the a axis by inter-molecular C-H⋯O hydrogen bonds and π-π stacking inter-actions, with centroid-centroid distances of 3.592 (5) Å.
Figures
References
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
-
- Bruker (1997). SMART, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cavalca, L., Gaetani, A., Mangia, A. & Pelizzi, G. (1970). Gazz. Chim. Ital.100, 629–638.
-
- Clerici, F., Gelmi, M. L., Pellegrino, S. & Pocar, D. (2007). Top. Heterocycl. Chem.9, 179–264.
-
- Farrugia, L. J. (1997). J. Appl. Cryst.30, 565.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
