Poly[[di-μ(3)-nicotinato-hemi-μ(4)-oxalato-hemi-μ(2)-oxalato-neodymium(III)silver(I)] dihydrate]
- PMID: 21582099
- PMCID: PMC2968511
- DOI: 10.1107/S1600536809005947
Poly[[di-μ(3)-nicotinato-hemi-μ(4)-oxalato-hemi-μ(2)-oxalato-neodymium(III)silver(I)] dihydrate]
Abstract
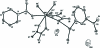
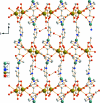
The asymmetric unit of the title compound, {[AgNd(C(6)H(4)NO(2))(2)(C(2)O(4))]·2H(2)O}(n), contains one Nd(III) atom, one Ag(I) atom, one oxalate ligand, two nicotinate ligands and two uncoordinated water mol-ecules. The Nd(III) atom is eight-coordinated in a distorted square-anti-prismatic coordination geometry by four O atoms from two oxalate ligands and four O atoms from four nicotinate ligands. The Ag(I) atom has a T-shaped configuration, defined by two N atoms from two nicotinate ligands and one O atom from one oxalate ligand. The nicotinate and oxalate ligands link the Nd and Ag atoms into a three-dimensional coordination framework. O-H⋯O and O-H⋯N hydrogen bonds donated by water mol-ecules are observed in the crystal.
Figures
Similar articles
-
Poly[[hemi-μ(4)-oxalato-hemi-μ(2)-oxalato-bis-(μ(3)-pyrazine-2-carboxyl-ato)neodymium(III)silver(I)] monohydrate].Acta Crystallogr Sect E Struct Rep Online. 2009 Jun 27;65(Pt 7):m833-4. doi: 10.1107/S160053680902128X. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21582751 Free PMC article.
-
Poly[[di-μ(3)-nicotinato-μ(3)-oxalato-samarium(III)silver(I)] dihydrate].Acta Crystallogr Sect E Struct Rep Online. 2009 Aug 19;65(Pt 9):m1105. doi: 10.1107/S1600536809032115. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21577452 Free PMC article.
-
Poly[[hemi-μ(4)-oxalato-hemi-μ(2)-oxalato-bis-(μ(3)-pyrazine-2-carboxyl-ato)erbium(III)silver(I)] monohydrate].Acta Crystallogr Sect E Struct Rep Online. 2009 Jul 31;65(Pt 8):m1018-9. doi: 10.1107/S1600536809029742. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21583314 Free PMC article.
-
catena-Poly[[tetra-μ(3)-isonicotinato-μ(3)-oxalato-μ(2)-oxalato-disamarium(III)disilver(I)] dihydrate].Acta Crystallogr Sect E Struct Rep Online. 2009 Dec 24;66(Pt 1):m88-9. doi: 10.1107/S1600536809054208. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21579979 Free PMC article.
-
Poly[[diaqua-bis-(μ-oxalato-κ(4)O(1),O(2):O(1'),O(2'))bis-(μ(3)-5-oxidopyridin-1-ium-3-carboxyl-ato-κ(3)O(3):O(3'):O(5))diholmium(III)] dihydrate].Acta Crystallogr Sect E Struct Rep Online. 2012 Sep 1;68(Pt 9):m1146-7. doi: 10.1107/S1600536812032916. Epub 2012 Aug 4. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22969449 Free PMC article.
References
-
- Arnold, D. I., Cotton, F. A., Matonic, J. H. & Murillo, C. A. (1997). Polyhedron, 16, 1837–1841.
-
- Barbour, L. J. (2006). Chem. Commun. pp. 1163–1168. - PubMed
-
- Brandenburg, K. & Putz, H. (1999). DIAMOND Crystal Impact GbR, Bonn, Germany.
-
- Bruker (2007). APEX2 and SAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cheng, J. W., Zheng, S. T. & Yang, G. Y. (2007a). Dalton Trans. pp. 4059–4066. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
