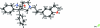Darifenacin hydro-bromide
- PMID: 21583148
- PMCID: PMC2969553
- DOI: 10.1107/S1600536809017085
Darifenacin hydro-bromide
Abstract
In the title compound {systematic name: (S)-3-[(aminocar-bonyl)diphenylmethyl]-1-[2-(2,3-di-hy-dro-benzofuran-5-yl)ethyl]pyrrolidinium bromide}, C(28)H(31)N(2)O(2) (+)·Br(-), the pyrrolidine rings adopts an envelope conformation. The two phenyl rings make a dihedral angle of 72.5 (1)°. The four coplanar atoms of the pyrrolidine ring makes dihedral angles of 33.1 (2) and 82.8 (2)° with the two phenyl rings. The mol-ecular conformation is influenced by a C-H⋯O inter-action. In the crystal packing, there are two N-H⋯Br hydrogen bonds running in opposite directions. They appear to form C(10) and C(9) chain motifs in the unit cell. In addition, the mol-ecular packing is further stabilized by C-H⋯Br and C-H⋯O hydrogen bonds. The C atom bonded to the benzofuran ring system is disordered in a 0.66:0.34 ratio.
Figures
Similar articles
-
Ethyl 1-[2-(1,3-benzoxazol-2-ylsulfan-yl)acet-yl]-4-hy-droxy-2,6-diphenyl-1,2,5,6-tetra-hydro-pyridine-3-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2011 Jul 1;67(Pt 7):o1731. doi: 10.1107/S1600536811022744. Epub 2011 Jun 18. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21837120 Free PMC article.
-
Methyl 3-(4-bromo-phen-yl)-2-(1H-indol-3-ylmeth-yl)-5-[1-(4-methoxy-phen-yl)-4-oxo-2-phenyl-azetidin-2-yl]-4-nitro-pyrrolidine-2-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2008 May 17;64(Pt 6):o1095-6. doi: 10.1107/S1600536808014190. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21202610 Free PMC article.
-
Ethyl 4-hy-droxy-6-(4-hy-droxy-phen-yl)-4-trifluoro-methyl-2-sulfanyl-idene-1,3-diazinane-5-carboxyl-ate ethanol monosolvate.Acta Crystallogr Sect E Struct Rep Online. 2011 May 1;67(Pt 5):o1280-1. doi: 10.1107/S1600536811015376. Epub 2011 Apr 29. Acta Crystallogr Sect E Struct Rep Online. 2011. PMID: 21754562 Free PMC article.
-
3-[Hy-droxy(3-meth-oxy-phen-yl)methyl-idene]-2-(2-oxo-2-phenyl-eth-yl)-3,4-dihydro-2H-1λ(6),2-benzothia-zine-1,1,4-trione.Acta Crystallogr Sect E Struct Rep Online. 2012 Apr 1;68(Pt 4):o978-9. doi: 10.1107/S1600536812009002. Epub 2012 Mar 7. Acta Crystallogr Sect E Struct Rep Online. 2012. PMID: 22590032 Free PMC article.
-
Ethyl 4-(2-bromo-5-fluoro-phen-yl)-6-methyl-1-phenyl-2-thioxo-1,2,3,4-tetra-hydro-pyrimidine-5-carboxyl-ate.Acta Crystallogr Sect E Struct Rep Online. 2008 Jul 19;64(Pt 8):o1526-7. doi: 10.1107/S1600536808021685. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21203233 Free PMC article.
Cited by
-
On modelling disordered crystal structures through restraints from molecule-in-cluster computations, and distinguishing static and dynamic disorder.IUCrJ. 2021 Feb 18;8(Pt 2):305-318. doi: 10.1107/S2052252521000531. eCollection 2021 Mar 1. IUCrJ. 2021. PMID: 33708406 Free PMC article.
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–S19.
-
- Bruker (2001). SMART andSAINT Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Chapple, C. R. (2004). Expert Opin. Investig. Drugs, 13, 1493–1500. - PubMed
-
- Cremer, D. & Pople, J. A. (1975). J. Am. Chem. Soc 97, 1354–1358.
-
- Croom, K. F. & Keating, G. M. (2004). Drugs Aging, 21, 885-892. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous