catena-Poly[[(ethanol-κO)[3-(1-phenyl-1H-pyrazol-3-yl)benzoic acid-κO]lithium]-μ-3-(1-phenyl-1H-pyrazol-3-yl)benzoato-κO:O']
- PMID: 21583374
- PMCID: PMC2977228
- DOI: 10.1107/S1600536809026385
catena-Poly[[(ethanol-κO)[3-(1-phenyl-1H-pyrazol-3-yl)benzoic acid-κO]lithium]-μ-3-(1-phenyl-1H-pyrazol-3-yl)benzoato-κO:O']
Abstract
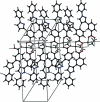
The asymmetric unit of the title polymeric compound, [Li(2)(C(16)H(11)N(2)O(2))(2)(C(16)H(12)N(2)O(2))(2)(CH(3)CH(2)OH)(2)](n), contains two Li(I) ions, two 3-(1-phenyl-1H-pyrazol-3-yl)benzoate ions, two 3-(1-phenyl-1H-pyrazol-3-yl)benzoic acid mol-ecules and two ethanol mol-ecules. In the crystal structure, each of the two Li(I) ions has a distorted tetra-hedral geometry, coordinated by two carboxyl-ate O atoms, one carboxyl O atom and one ethanol O atom. The carboxyl-ate group bridges the Li(I) ions, forming a one-dimensional polymeric chain along [100]. The crystal structure is further stabilized by O-H⋯O and C-H⋯N hydrogen bonding, and π-π inter-actions with centroid-centroid distances in the range 3.6534 (13)-3.8374 (13) Å.
Figures
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Bruker (2005). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
-
- Di Marzo, V., Bifulco, M. & De Petrocellis, L. (2004). Nat. Rev. Drug Discov.3, 771–784. - PubMed
-
- Fischer, A. (2005). Acta Cryst. E61, m320–m322.
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous