2,2-Diphenyl-benzo[c]quinoline-1-ox-yl
- PMID: 21583864
- PMCID: PMC2977728
- DOI: 10.1107/S1600536809013476
2,2-Diphenyl-benzo[c]quinoline-1-ox-yl
Abstract
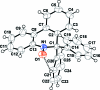
In the title compound, C(25)H(18)NO, a stable phenanthridinic nitroxide, the ring containing the nitroxide function assumes a twist-boat conformation and the dihedral angle formed by adjacent benzene rings is 21.78 (5)°. The phenyl substituents at position 2 are approximately orthogonal to each other, forming a dihedral angle of 81.04 (4)°. The crystal structure is stabilized by an intra-molecular C-H⋯O hydrogen bond and by C-H⋯π inter-actions.
Figures
References
-
- Allen, F. H. (2002). Acta Cryst. B58, 380–388. - PubMed
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
-
- Arends, I., Li, Y.-X. & Sheldon, A. R. (2006). Biocatal. Biotransform.24, 443–448.
-
- Bailly, B., Donnenwirth, A.-C., Bartholome, C., Beyou, E. & Bourgeat-Lami, E. (2006). J. Nanomater. pp. 1–10.
-
- Bruker (1998). SMART, SAINT-Plus and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
