4-(3-Methyl-anilino)-N-[N-(1-methyl-ethyl)carbamo-yl]pyridinium-3-sulfon-amidate (torasemide) methanol 0.25-solvate 0.25-hydrate
- PMID: 21584012
- PMCID: PMC2977669
- DOI: 10.1107/S160053680901160X
4-(3-Methyl-anilino)-N-[N-(1-methyl-ethyl)carbamo-yl]pyridinium-3-sulfon-amidate (torasemide) methanol 0.25-solvate 0.25-hydrate
Abstract
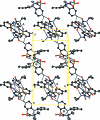
The title compound, C(16)H(20)N(4)O(3)S·0.25CH(4)O·0.25H(2)O, is a hydrate/methanol solvate of torasemide, a diuretic drug used in the treatment of hypertension. The asymmetric unit contains two torasemide mol-ecules and half-occupied methanol and water mol-ecules. It is isomorphous with the previously reported nonsolvated T-II form of torasemide. The water mol-ecules contribute to the stability of the structure by participating in an extensive system of O-H⋯O hydrogen bonds; N-H⋯N and N-H⋯O hydrogen bonds are also present. Both asymmetric mol-ecules of torasemide form inversion dimers in the crystal.
Figures



References
-
- Altomare, A., Burla, M. C., Camalli, M., Cascarano, G. L., Giacovazzo, C., Guagliardi, A., Moliterni, A. G. G., Polidori, G. & Spagna, R. (1999). J. Appl. Cryst.32, 115–119.
-
- Broekhuysen, J., Deger, F., Douchamps, J., Ducarne, H. & Herchuelz, A. (1986). Eur. J. Clin. Pharmacol.31 Suppl, 29–34. - PubMed
-
- Cosin, J. & Diez, J. (2002). Eur. J. Heart Failure, 4, 507–513. - PubMed
-
- Danilovski, A., Filić, D., Orešić, M. & Dumić, M. (2001). Croat. Chim. Acta, 74, 103–120.
-
- Dupont, L., Campsteyn, H., Lamotte, J. & Vermeire, M. (1978). Acta Cryst. B34, 2659–2662.
LinkOut - more resources
Full Text Sources
