Sinomenine protects against ischaemic brain injury: involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels
- PMID: 21585344
- PMCID: PMC3221099
- DOI: 10.1111/j.1476-5381.2011.01487.x
Sinomenine protects against ischaemic brain injury: involvement of co-inhibition of acid-sensing ion channel 1a and L-type calcium channels
Abstract
Background and purpose: Sinomenine (SN), a bioactive alkaloid, has been utilized clinically to treat rheumatoid arthritis in China. Our preliminary experiments indicated that it could protect PC12 cells from oxygen-glucose deprivation-reperfusion (OGD-R), we thus investigated the possible effects of SN on cerebral ischaemia and the related mechanism.
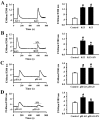
Experimental approach: Middle cerebral artery occlusion in rats was used as an animal model of ischaemic stroke in vivo. The mechanisms of the effects of SN were investigated in vitro using whole-cell patch-clamp recording, calcium imaging in PC12 cells and rat cortical neurons subjected to OGD-R.
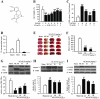
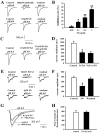
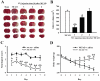
Key results: Pretreatment with SN (10 and 30 mg·kg(-1) , i.p.) significantly decreased brain infarction and the overactivation of calcium-mediated events in rats subjected to 2 h ischaemia followed by 24 h reperfusion. Extracellular application of SN inhibited the currents mediated by acid-sensing ion channel 1a and L-type voltage-gated calcium channels, in the rat cultured neurons, in a concentration-dependent manner. These inhibitory effects contribute to the neuroprotection of SN against OGD-R and extracellular acidosis-induced cytotoxicity. More importantly, administration of SN (30 mg·kg(-1) , i.p.) at 1 and 2 h after cerebral ischaemia also decreased brain infarction and improved functional recovery.
Conclusion and implications: SN exerts potent protective effects against ischaemic brain injury when administered before ischaemia or even after the injury. The inhibitory effects of SN on acid-sensing ion channel 1a and L-type calcium channels are involved in this neuroprotection.
© 2011 The Authors. British Journal of Pharmacology © 2011 The British Pharmacological Society.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

