Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR)
- PMID: 21586137
- PMCID: PMC3112409
- DOI: 10.1186/1477-7827-9-67
Dehydroepiandrosterone (DHEA) supplementation in diminished ovarian reserve (DOR)
Abstract
Background: With infertility populations in the developed world rapidly aging, treatment of diminished ovarian reserve (DOR) assumes increasing clinical importance. Dehydroepiandrosterone (DHEA) has been reported to improve pregnancy chances with DOR, and is now utilized by approximately one third of all IVF centers world-wide. Increasing DHEA utilization and publication of a first prospectively randomized trial now warrants a systematic review.
Methods: PubMed, Cochrane and Ovid Medline were searched between 1995 and 2010 under the following strategy: [<dehydroepiandrosterone or DHEA or androgens or testosterone > and <ovarian reserve or diminished ovarian reserve or ovarian function >]. Bibliographies of relevant publications were further explored for additional relevant citations. Since only one randomized study has been published, publications, independent of evidence levels and quality assessment, were reviewed.
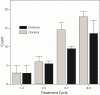
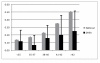
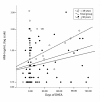
Results: Current best available evidence suggests that DHEA improves ovarian function, increases pregnancy chances and, by reducing aneuploidy, lowers miscarriage rates. DHEA over time also appears to objectively improve ovarian reserve. Recent animal data support androgens in promoting preantral follicle growth and reduction in follicle atresia.
Discussion: Improvement of oocyte/embryo quality with DHEA supplementation potentially suggests a new concept of ovarian aging, where ovarian environments, but not oocytes themselves, age. DHEA may, thus, represent a first agent beneficially affecting aging ovarian environments. Others can be expected to follow.
Figures


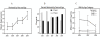
References
-
- Buster JE, Casson PR, Straughn AB, Dale D, Umstot ES, Chiamori N, Abraham GE. Postmenopausal steroid replacement with micronized dehydroepiandrosterone: preliminary oral bioavailability and dose proportionality studies. Am J Obstet Gynecol. 1992;66:1163–1168. - PubMed
-
- Casson PR, Andersen RN, Herrod HG, Stentz FB, Straughn AB, Abraham GE, Buster JE. Oral dehydroepiandrosterone in physiologic doses modulates immune function in postmenopausal women. Am J Obstet Gynecol. 1993;169:1536–1539. - PubMed
-
- Harding G, Mak YT, Evans B, Cheung J, MacDonald D, Hampson G. The effects of dexamethasone and dehydroepiandrosterone (DHEA) on cytokines and receptor expression in a human osteoblastic cell line: potential steroid-sparing role of DHEA. Cytokine. 2006;36:57–68. doi: 10.1016/j.cyto.2006.10.012. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

