Fruit ripening in Vitis vinifera: spatiotemporal relationships among turgor, sugar accumulation, and anthocyanin biosynthesis
- PMID: 21586429
- PMCID: PMC3153685
- DOI: 10.1093/jxb/err150
Fruit ripening in Vitis vinifera: spatiotemporal relationships among turgor, sugar accumulation, and anthocyanin biosynthesis
Abstract
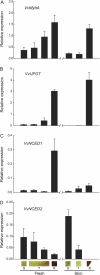
This study reports the first observations indicating the spatiotemporal relationships among genetic and physiological aspects of ripening in the berry of Vitis vinifera. At the onset of ripening in the red flesh variety Alicante Bouschet, colour development began in the flesh at the stylar end of the fruit and progressed toward the pedicel end flesh and into the skin. Tissue solute potential and cell turgor also decreased first in the flesh. The decrease in flesh solute potential was due to accumulation of sugars, glucose and fructose, an accumulation that is integral to ripening. Expression of the anthocyanin biosynthesis-related genes VvMybA and VvUFGT was linearly related to the decrease in solute potential. Expression of VvMybA, and to a lesser extent VvUFGT, was correspondingly low in green tissue, higher in the red, stylar end flesh of berries beginning to ripen, and greatest in red berries. In contrast, expression of the abscisic acid biosynthesis-related genes VvNCED1 and VvNCED2 was not correlated with the other spatiotemporal aspects of the onset of ripening. These results, together with earlier work showing that sugar accumulation and acid loss also begin in the stylar flesh in other varieties, indicate that ripening in the grape berry originates in the stylar end flesh.
Figures




References
-
- Ageorges A, Fernandez L, Vialet S, Merdinoglu D, Terrier N, Romieu C. Four specific isogenes of the anthocyanin metabolic pathway are systematically co-expressed with the red colour of grape berries. Plant Science. 2006;170:372–383.
-
- Boss PK, Davies C, Robinson SP. Expression of anthocyanin biosynthesis pathway genes in red and white grapes. Plant Molecular Biology. 1996b;32:565–569. - PubMed

