The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents
- PMID: 21586439
- PMCID: PMC3128235
- DOI: 10.1136/ard.2010.146365
The comparative risk of serious infections among rheumatoid arthritis patients starting or switching biological agents
Abstract
Background: It is unclear whether anti-tumour necrosis factor alpha and biological agents with different mechanisms of action have similar safety. This study evaluated the incidence of hospitalised infections among rheumatoid arthritis (RA) patients starting or switching various biological agents.
Methods: Using a database from a large US healthcare organisation from January 2005 to August 2009, the authors identified enrollees with RA and their treatment episodes entailing the new use of a biological agent, stratified by no biological use in the previous year ('biological-free') or switching from a different biological agent ('switchers'). Outcomes were hospitalised infections identified using previously validated algorithms. Proportional hazards models estimated the hazard ratio of hospitalised infections, comparing each biological agent with infliximab.
Results: Among 7847 biological treatment episodes, 63% were for biological-free patients and 37% for switchers. There were 364 hospitalised infections. Rates of hospitalised infection among biological-free patients and switchers were 4.6 and 7.0 per 100 person-years, respectively (p<0.0001). After multivariable adjustment controlling for biological-free/switcher status and other infection-related factors and compared with infliximab, users of abatacept (HR 0.68, 95% CI 0.48 to 0.96), adalimumab (HR 0.52, 0.39 to 0.71), etanercept (HR 0.64, 0.49 to 0.84) and rituximab (HR 0.81, 0.55 to 1.20) had lower rates of hospitalised infection. Patient risk factors contributed more to the risk of infection than did the risk associated with specific biological therapies.
Conclusion: The rate of hospitalised infections among RA patients was highest for infliximab. Most of the variability in patients' risk of infection was driven by factors other than biological agent exposure.
Figures
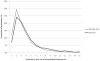
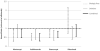
References
-
- Bergman GJ, Hochberg MC, Boers M, et al. Indirect comparison of tocilizumab and other biologic agents in patients with rheumatoid arthritis and inadequate response to disease-modifying antirheumatic drugs. Semin Arthritis Rheum. 2010;39:425–41. - PubMed
-
- Salliot C, Finckh A, Katchamart W, et al. Indirect comparisons of the efficacy of biological antirheumatic agents in rheumatoid arthritis in patients with an inadequate response to conventional disease-modifying antirheumatic drugs or to an anti-tumour necrosis factor agent: a meta-analysis. Ann Rheum Dis. 2011;70:266–71. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

