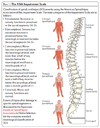
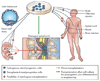
Will stem cell therapies be safe and effective for treating spinal cord injuries?
- PMID: 21586446
- PMCID: PMC5583428
- DOI: 10.1093/bmb/ldr013
Will stem cell therapies be safe and effective for treating spinal cord injuries?
Abstract
Introduction: A large number of different cells including embryonic and adult stem cells have been transplanted into animal models of spinal cord injury, and in many cases these procedures have resulted in modest sensorimotor benefits. In October 2010 the world's first clinical trial using human embryonic stem cells began, using stem cells converted into oligodendrocyte precursor cells.
Sources of data: In this review we examine some of the publically available preclinical evidence that some of these cell types improve outcome in animal models of spinal cord injury. Much evidence is not available for public scrutiny, however, being private commercial property of various stem cell companies.
Areas of agreement: Transplantation of many different types of stem and progenitor cell enhances spontaneous recovery of function when transplanted acutely after spinal cord injury in animal models. AREAS OF DISAGREEMENT: The common mechanism(s) whereby the generic procedure of cellular transplantation enhances recovery of function are not well understood, although a range of possibilities are usually cited (including preservation of tissue, remyelination, axon sprouting, glial cell replacement). Only in exceptional cases has it been shown that functional recovery depends causally on the survival and differentiation of the transplanted cells. There is no agreement about the optimal cell type for transplantation: candidate stem cells have not yet been compared with each other or with other cell types (e.g. autologous Schwann cells) in a single study.
Areas timely for developing research: Transplantation of cells into animals with a long lifespan is important to determine whether or not tumours will eventually form. It will also be important to determine whether long-term survival of cells is required for functional recovery, and if so, how many are optimal.
Figures
References
-
- Steeves JD, Fawcett JW, Tuszynski MH, Lammertse DP, Curt AEP, Ditunno JF, Ellaway PH, Fehlings MG, Guest JD, Kleitman N, Bartlett PF, et al. Experimental treatments for spinal cord injury: what you should know if you are considering participation in a clinical trial. 2007
-
- Thuret S, Moon LD, Gage FH. Therapeutic interventions after spinal cord injury. Nat Rev Neurosci. 2006;7:628–43. - PubMed
-
- Fawcett JW, Curt A, Steeves JD, Coleman WP, Tuszynski MH, Lammertse D, Bartlett PF, Blight AR, Dietz V, Ditunno J, Dobkin BH, et al. Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord. 2007;45:190–205. - PubMed
-
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Beattie MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nature Medicine. 1997;3:73–76. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials