Malignant tumours of the small intestine: a review of histopathology, multidetector CT and MRI aspects
- PMID: 21586504
- PMCID: PMC3473441
- DOI: 10.1259/bjr/20673379
Malignant tumours of the small intestine: a review of histopathology, multidetector CT and MRI aspects
Abstract
Small bowel neoplasms, including adenocarcinoma, carcinoid tumour, lymphoma and gastrointestinal stromal tumours, represent a small percentage of gastrointestinal cancers, yet are among those with the poorest prognosis compared with other gastrointestinal malignancies. Unclear clinical scenarios and difficult radiological diagnosis often delay treatment with negative effects on patient survival. Recently, multidetector CT (MDCT) and MRI have been introduced as feasible and accurate diagnostic techniques for the identification and staging of small bowel neoplasms. These techniques are gradually replacing conventional barium radiography as the tool of choice. However, the inherent technical and physiological challenges of small bowel imaging require a familiarity with patient preparation and scan protocols. Adequate knowledge of the histopathology and natural evolution of small bowel neoplasms is also important for differential diagnosis. The aim of this article is to review MDCT and MRI protocols for the evaluation of small bowel tumours and to provide a concise yet comprehensive guide to the most relevant imaging features relative to histopathology.
Figures



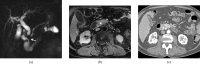

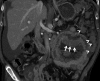


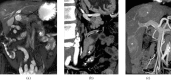



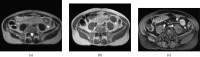





Similar articles
-
Multidetector-row computed tomography and 3-dimensional computed tomography imaging of small bowel neoplasms: current concept in diagnosis.J Comput Assist Tomogr. 2004 Jan-Feb;28(1):106-16. doi: 10.1097/00004728-200401000-00019. J Comput Assist Tomogr. 2004. PMID: 14716243 Review.
-
Radiopathological review of small bowel carcinoid tumours.J Med Imaging Radiat Oncol. 2009 Feb;53(1):1-12. doi: 10.1111/j.1754-9485.2009.02031.x. J Med Imaging Radiat Oncol. 2009. PMID: 19453523 Review.
-
Cross-sectional imaging of small bowel malignancies.Can Assoc Radiol J. 2012 Aug;63(3):215-21. doi: 10.1016/j.carj.2010.10.001. Epub 2011 Aug 27. Can Assoc Radiol J. 2012. PMID: 21873023 Review.
-
Diagnosis and staging of small bowel tumours.Cancer Imaging. 2006 Dec 29;6(1):209-12. doi: 10.1102/1470-7330.2006.0031. Cancer Imaging. 2006. PMID: 17208678 Free PMC article. Review.
-
Radiological findings in primary malignant tumours of the small intestine.Ann Clin Res. 1971 Feb;3(1):16-21. Ann Clin Res. 1971. PMID: 5107813 No abstract available.
Cited by
-
Double-balloon enteroscopy in small bowel tumors: a Chinese single-center study.World J Gastroenterol. 2013 Jun 21;19(23):3665-71. doi: 10.3748/wjg.v19.i23.3665. World J Gastroenterol. 2013. PMID: 23801870 Free PMC article.
-
Duodenal tumors on cross-sectional imaging with emphasis on multidetector computed tomography: a pictorial review.Diagn Interv Radiol. 2020 May;26(3):193-199. doi: 10.5152/dir.2019.19241. Diagn Interv Radiol. 2020. PMID: 32209505 Free PMC article. Review.
-
Primary Gastro-Intestinal Lymphoma and Gastro-Intestinal Adenocarcinoma: An Initial Study of CT Texture Analysis as Quantitative Biomarkers for Differentiation.Life (Basel). 2021 Mar 23;11(3):264. doi: 10.3390/life11030264. Life (Basel). 2021. PMID: 33806817 Free PMC article.
-
De novo distal terminal ileum adenocarcinoma mimicking Crohn's disease and diagnostic challenges in imaging: a case series.BJR Case Rep. 2021 Jun 24;7(6):20210103. doi: 10.1259/bjrcr.20210103. eCollection 2022 Mar. BJR Case Rep. 2021. PMID: 35300226 Free PMC article.
-
Small intestine leiomyosarcoma mimicking gastrointestinal stromal tumour.BMJ Case Rep. 2021 Sep 13;14(9):e244381. doi: 10.1136/bcr-2021-244381. BMJ Case Rep. 2021. PMID: 34518183 Free PMC article.
References
-
- Martin RG. Malignant tumors of the small intestine. Surg Clin North Am 1986;66:779–85 - PubMed
-
- North JH, Pack MS. Malignant tumors of the small intestine: a review of 144 cases. Am Surg 2000;66:46–51 - PubMed
-
- Horner MJ, Ries LAG, Krapcho M, Neyman N, Aminou R, Howlader N, et al., editors. SEER cancer statistics review, 1975-2006, National Cancer Institute: Bethesda, MD. Available at: http://seer.cancer.gov/csr/1975_2006/
-
- Yamagami H, Oshitani N, Hosomi S, Suekane T, Kamata N, Sogawa M, et al. Usefulness of double-balloon endoscopy in the diagnosis of malignant small-bowel tumors. Clin Gastroenterol Hepatol 2008;6:1202–5 - PubMed
-
- Maglinte DD, O'Connor K, Bessette J, Chernish SM, Kelvin FM. The role of the physician in the late diagnosis of primary malignant tumors of the small intestine. Am J Gastroenterol 1991;86:304–8 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

