CHRNA5 as negative regulator of nicotine signaling in normal and cancer bronchial cells: effects on motility, migration and p63 expression
- PMID: 21586512
- PMCID: PMC3165122
- DOI: 10.1093/carcin/bgr090
CHRNA5 as negative regulator of nicotine signaling in normal and cancer bronchial cells: effects on motility, migration and p63 expression
Abstract
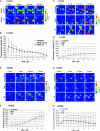
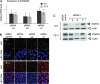
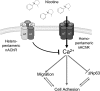
Genome-wide association studies have linked lung cancer risk with a region of chromosome 15q25.1 containing CHRNA3, CHRNA5 and CHRNB4 encoding α3, α5 and β4 subunits of nicotinic acetylcholine receptors (nAChR), respectively. One of the strongest associations was observed for a non-silent single-nucleotide polymorphism at codon 398 in CHRNA5. Here, we have used pharmacological (antagonists) or genetic (RNA interference) interventions to modulate the activity of CHRNA5 in non-transformed bronchial cells and in lung cancer cell lines. In both cell types, silencing CHRNA5 or inhibiting receptors containing nAChR α5 with α-conotoxin MII exerted a nicotine-like effect, with increased motility and invasiveness in vitro and increasing calcium influx. The effects on motility were enhanced by addition of nicotine but blocked by inhibiting CHRNA7, which encodes the homopentameric receptor α7 subunit. Silencing CHRNA5 also decreased the expression of cell adhesion molecules P120 and ZO-1 in lung cancer cells as well as the expression of DeltaNp63α in squamous cell carcinoma cell lines. These results demonstrate a role for CHRNA5 in modulating adhesion and motility in bronchial cells, as well as in regulating p63, a potential oncogene in squamous cell carcinoma.
Figures


Similar articles
-
Contribution of Variants in CHRNA5/A3/B4 Gene Cluster on Chromosome 15 to Tobacco Smoking: From Genetic Association to Mechanism.Mol Neurobiol. 2016 Jan;53(1):472-484. doi: 10.1007/s12035-014-8997-x. Epub 2014 Dec 5. Mol Neurobiol. 2016. PMID: 25471942 Review.
-
Nicotine activates cell-signaling pathways through muscle-type and neuronal nicotinic acetylcholine receptors in non-small cell lung cancer cells.Pulm Pharmacol Ther. 2007;20(6):629-41. doi: 10.1016/j.pupt.2006.07.001. Epub 2006 Aug 18. Pulm Pharmacol Ther. 2007. PMID: 17015027
-
Reciprocal activation of α5-nAChR and STAT3 in nicotine-induced human lung cancer cell proliferation.J Genet Genomics. 2017 Jul 20;44(7):355-362. doi: 10.1016/j.jgg.2017.03.003. Epub 2017 Mar 21. J Genet Genomics. 2017. PMID: 28750889
-
β4-Nicotinic Receptors Are Critically Involved in Reward-Related Behaviors and Self-Regulation of Nicotine Reinforcement.J Neurosci. 2020 Apr 22;40(17):3465-3477. doi: 10.1523/JNEUROSCI.0356-19.2020. Epub 2020 Mar 17. J Neurosci. 2020. PMID: 32184221 Free PMC article.
-
From smoking to lung cancer: the CHRNA5/A3/B4 connection.Oncogene. 2010 Sep 2;29(35):4874-84. doi: 10.1038/onc.2010.256. Epub 2010 Jun 28. Oncogene. 2010. PMID: 20581870 Free PMC article. Review.
Cited by
-
Role of α7-nicotinic acetylcholine receptor in nicotine-induced invasion and epithelial-to-mesenchymal transition in human non-small cell lung cancer cells.Oncotarget. 2016 Sep 13;7(37):59199-59208. doi: 10.18632/oncotarget.10498. Oncotarget. 2016. PMID: 27409670 Free PMC article.
-
Genome-wide association studies identify CHRNA5/3 and HTR4 in the development of airflow obstruction.Am J Respir Crit Care Med. 2012 Oct 1;186(7):622-32. doi: 10.1164/rccm.201202-0366OC. Epub 2012 Jul 26. Am J Respir Crit Care Med. 2012. PMID: 22837378 Free PMC article.
-
Nicotine activates YAP1 through nAChRs mediated signaling in esophageal squamous cell cancer (ESCC).PLoS One. 2014 Mar 12;9(3):e90836. doi: 10.1371/journal.pone.0090836. eCollection 2014. PLoS One. 2014. PMID: 24621512 Free PMC article.
-
Retroelement co-option disrupts the cancer transcriptional programme.Genome Med. 2025 May 7;17(1):48. doi: 10.1186/s13073-025-01479-9. Genome Med. 2025. PMID: 40336011 Free PMC article.
-
Mining and validating the expression pattern and prognostic value of acetylcholine receptors in non-small cell lung cancer.Medicine (Baltimore). 2019 May;98(20):e15555. doi: 10.1097/MD.0000000000015555. Medicine (Baltimore). 2019. PMID: 31096457 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

