Characterization of RIN3 as a guanine nucleotide exchange factor for the Rab5 subfamily GTPase Rab31
- PMID: 21586568
- PMCID: PMC3129215
- DOI: 10.1074/jbc.M110.172445
Characterization of RIN3 as a guanine nucleotide exchange factor for the Rab5 subfamily GTPase Rab31
Abstract
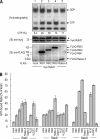
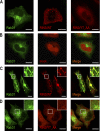
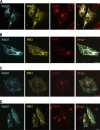
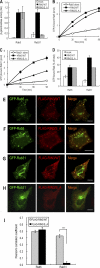
The small GTPase Rab5, which cycles between GDP-bound inactive and GTP-bound active forms, plays essential roles in membrane budding and trafficking in the early endocytic pathway. Rab5 is activated by various vacuolar protein sorting 9 (VPS9) domain-containing guanine nucleotide exchange factors. Rab21, Rab22, and Rab31 (members of the Rab5 subfamily) are also involved in the trafficking of early endosomes. Mechanisms controlling the activation Rab5 subfamily members remain unclear. RIN (Ras and Rab interactor) represents a family of multifunctional proteins that have a VPS9 domain in addition to Src homology 2 (SH2) and Ras association domains. We investigated whether RIN family members act as guanine nucleotide exchange factors (GEFs) for the Rab5 subfamily on biochemical and cell morphological levels. RIN3 stimulated the formation of GTP-bound Rab31 in cell-free and in cell GEF activity assays. RIN3 also formed enlarged vesicles and tubular structures, where it colocalized with Rab31 in HeLa cells. In contrast, RIN3 did not exhibit any apparent effects on Rab21. We also found that serine to alanine substitutions in the sequences between SH2 and RIN family homology domain of RIN3 specifically abolished its GEF action on Rab31 but not Rab5. We examined whether RIN3 affects localization of the cation-dependent mannose 6-phosphate receptor (CD-MPR), which is transported between trans-Golgi network and endocytic compartments. We found that RIN3 partially translocates CD-MPR from the trans-Golgi network to peripheral vesicles and that this is dependent on its Rab31-GEF activity. These results indicate that RIN3 specifically acts as a GEF for Rab31.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

