The Mycobacterium tuberculosis early secreted antigenic target of 6 kDa inhibits T cell interferon-γ production through the p38 mitogen-activated protein kinase pathway
- PMID: 21586573
- PMCID: PMC3129230
- DOI: 10.1074/jbc.M111.234062
The Mycobacterium tuberculosis early secreted antigenic target of 6 kDa inhibits T cell interferon-γ production through the p38 mitogen-activated protein kinase pathway
Abstract
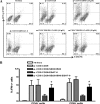
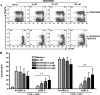
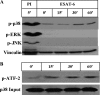
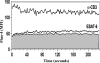
We reported previously that the early secreted antigenic target of 6 kDa (ESAT-6) from Mycobacterium tuberculosis directly inhibits human T cell IFN-γ production and proliferation in response to stimulation with anti-CD3 and anti-CD28. To determine the mechanism of this effect, we treated T cells with kinase inhibitors before stimulation with ESAT-6. Only the p38 MAPK inhibitor, SB203580, abrogated ESAT-6-mediated inhibition of IFN-γ production in a dose-dependent manner. SB203580 did not reverse ESAT-6-mediated inhibition of IL-17 and IL-10 production, suggesting a specific effect of SB203580 on IFN-γ production. SB203580 did not act through inhibition of AKT (PKB) as an AKT inhibitor did not affect ESAT-6 inhibition of T cell IFN-γ production and proliferation. ESAT-6 did not reduce IFN-γ production by expanding FoxP3(+) T regulatory cells. Incubation of T cells with ESAT-6 induced phosphorylation and increased functional p38 MAPK activity, but not activation of ERK or JNK. Incubation of peripheral blood mononuclear cells with ESAT-6 induced activation of p38 MAPK, and inhibition of p38 MAPK with SB203580 reversed ESAT-6 inhibition of M. tuberculosis-stimulated IFN-γ production by peripheral blood mononuclear cells from subjects with latent tuberculosis infection. Silencing of p38α MAPK with siRNA rendered T cells resistant to ESAT-6 inhibition of IFN-γ production. Taken together, our results demonstrate that ESAT-6 inhibits T cell IFN-γ production in a p38 MAPK-dependent manner.
Figures
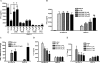
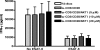
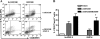
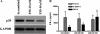
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

