STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis
- PMID: 21586612
- PMCID: PMC3693754
- DOI: 10.1158/0008-5472.CAN-11-0908
STAT3 plays a critical role in KRAS-induced pancreatic tumorigenesis
Abstract
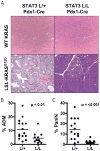
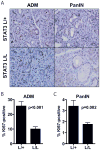
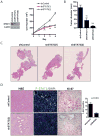
The STAT3 transcription factor is an important regulator of stem cell self-renewal, cancer cell survival, and inflammation. In the pancreas, STAT3 is dispensable for normal development, whereas the majority of pancreatic ductal adenocarcinomas (PDAC) show constitutive activation of STAT3, suggesting its potential as a therapeutic target in this cancer. Here, we sought to define the mechanisms of STAT3 activation and its functional importance in PDAC pathogenesis. Large-scale screening of cancer cell lines with a JAK2 inhibitor that blocks STAT3 function revealed a more than 30-fold range in sensitivity in PDAC, and showed a close correlation of sensitivity with levels of tyrosine-phosphorylated STAT3 and of the gp130 receptor, an upstream signaling component. Correspondingly, upregulation of the IL6/LIF-gp130 pathway accounted for the strong STAT3 activation in PDAC subsets. To define functions of STAT3 in vivo, we developed mouse models that test the impact of conditional inactivation of STAT3 in KRAS-driven PDAC. We showed that STAT3 is required for the development of the earliest premalignant pancreatic lesions, acinar-to-ductal metaplasia (ADM) and pancreatic intraepithelial neoplasia (PanIN). Moreover, acute STAT3 inactivation blocked PDAC initiation in a second in vivo model. Our results show that STAT3 has critical roles throughout the course of PDAC pathogenesis, supporting the development of therapeutic approaches targeting this pathway. Moreover, our work suggests that gp130 and phospho-STAT3 expression may be effective biomarkers for predicting response to JAK2 inhibitors.
©2011 AACR.
Figures



References
-
- Warshaw AL, Fernández-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. - PubMed
-
- Hruban RH, van Mansfeld AD, Offerhaus GJ, van Weering DH, Allison DC, Goodman SN, et al. K-ras oncogene activation in adenocarcinoma of the human pancreas. A study of 82 carcinomas using a combination of mutant-enriched polymerase chain reaction analysis and allele-specific oligonucleotide hybridization. Am J Pathol. 1993;143:545–54. - PMC - PubMed
-
- Moskaluk CA, Hruban RH, Kern SE. p16 and K-ras gene mutations in the intraductal precursors of human pancreatic adenocarcinoma. Cancer Res. 1997;57:2140–3. - PubMed
-
- Rozenblum E, Schutte M, Goggins M, Hahn SA, Panzer S, Zahurak M, et al. Tumor-suppressive pathways in pancreatic carcinoma. Cancer Res. 1997 May 1;57(9):1731–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

