Imaging DNA damage in vivo using gammaH2AX-targeted immunoconjugates
- PMID: 21586614
- PMCID: PMC3130133
- DOI: 10.1158/0008-5472.CAN-10-4587
Imaging DNA damage in vivo using gammaH2AX-targeted immunoconjugates
Abstract
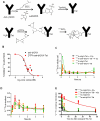
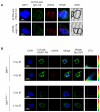
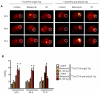
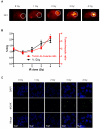
DNA damage responses (DDR) occur during oncogenesis and therapeutic responses to DNA damaging cytotoxic drugs. Thus, a real-time method to image DNA damage in vivo would be useful to diagnose cancer and monitor its treatment. Toward this end, we have developed fluorophore- and radioisotope-labeled immunoconjugates to target a DDR signaling protein, phosphorylated histone H2A variant H2AX (γH2AX), which forms foci at sites of DNA double-strand breaks. Anti-γH2AX antibodies were modified by the addition of diethylenetriaminepentaacetic acid (DTPA) to allow (111)In labeling or the fluorophore Cy3. The cell-penetrating peptide Tat (GRKKRRQRRRPPQGYG) was also added to the immunoconjugate to aid nuclear translocation. In irradiated breast cancer cells, confocal microscopy confirmed the expected colocalization of anti-γH2AX-Tat with γH2AX foci. In comparison with nonspecific antibody conjugates, (111)In-anti-γH2AX-Tat was retained longer in cells. Anti-γH2AX-Tat probes were also used to track in vivo DNA damage, using a mouse xenograft model of human breast cancer. After local X-ray irradiation or bleomycin treatment, the anti-γH2AX-Tat probes produced fluorescent and single photon emission computed tomography signals in the tumors that were proportionate to the delivered radiation dose and the amount of γH2AX present. Taken together, our findings establish the use of radioimmunoconjugates that target γH2AX as a noninvasive imaging method to monitor DNA damage, with many potential applications in preclinical and clinical settings.
©2011 AACR.
Figures


References
-
- Halazonetis TD, Gorgoulis VG, Bartek J. An oncogene-induced DNA damage model for cancer development. Science. 2008;319:1352–5. - PubMed
-
- Gorgoulis VG, Vassiliou LV, Karakaidos P, Zacharatos P, Kotsinas A, Liloglou T, et al. Activation of the DNA damage checkpoint and genomic instability in human precancerous lesions. Nature. 2005;434:907–13. - PubMed
-
- Sedelnikova OA, Bonner WM. GammaH2AX in cancer cells: a potential biomarker for cancer diagnostics, prediction and recurrence. Cell Cycle. 2006;5:2909–13. - PubMed
-
- Helleday T, Petermann E, Lundin C, Hodgson B, Sharma RA. DNA repair pathways as targets for cancer therapy. Nat Rev Cancer. 2008;8:193–204. - PubMed
-
- Qvarnstrom OF, Simonsson M, Johansson KA, Nyman J, Turesson I. DNA double strand break quantification in skin biopsies. Radiother Oncol. 2004;72:311–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

