Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers
- PMID: 21586636
- PMCID: PMC3111287
- DOI: 10.1073/pnas.1106536108
Structural basis for immunization with postfusion respiratory syncytial virus fusion F glycoprotein (RSV F) to elicit high neutralizing antibody titers
Abstract
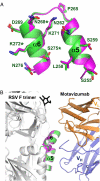
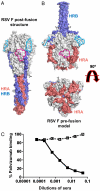
Respiratory syncytial virus (RSV), the main cause of infant bronchiolitis, remains a major unmet vaccine need despite more than 40 years of vaccine research. Vaccine candidates based on a chief RSV neutralization antigen, the fusion (F) glycoprotein, have foundered due to problems with stability, purity, reproducibility, and potency. Crystal structures of related parainfluenza F glycoproteins have revealed a large conformational change between the prefusion and postfusion states, suggesting that postfusion F antigens might not efficiently elicit neutralizing antibodies. We have generated a homogeneous, stable, and reproducible postfusion RSV F immunogen that elicits high titers of neutralizing antibodies in immunized animals. The 3.2-Å X-ray crystal structure of this substantially complete RSV F reveals important differences from homology-based structural models. Specifically, the RSV F crystal structure demonstrates the exposure of key neutralizing antibody binding sites on the surface of the postfusion RSV F trimer. This unanticipated structural feature explains the engineered RSV F antigen's efficiency as an immunogen. This work illustrates how structural-based antigen design can guide the rational optimization of candidate vaccine antigens.
Conflict of interest statement
Conflict of interest statement: The authors are Novartis shareholders and employees of Novartis Vaccines and Diagnostics.
Figures



Similar articles
-
Discovery of a Prefusion Respiratory Syncytial Virus F-Specific Monoclonal Antibody That Provides Greater In Vivo Protection than the Murine Precursor of Palivizumab.J Virol. 2017 Jul 12;91(15):e00176-17. doi: 10.1128/JVI.00176-17. Print 2017 Aug 1. J Virol. 2017. PMID: 28539438 Free PMC article.
-
Influence of Respiratory Syncytial Virus F Glycoprotein Conformation on Induction of Protective Immune Responses.J Virol. 2016 May 12;90(11):5485-5498. doi: 10.1128/JVI.00338-16. Print 2016 Jun 1. J Virol. 2016. PMID: 27009962 Free PMC article.
-
Effect of Previous Respiratory Syncytial Virus Infection on Murine Immune Responses to F and G Protein-Containing Virus-Like Particles.J Virol. 2019 Apr 17;93(9):e00087-19. doi: 10.1128/JVI.00087-19. Print 2019 May 1. J Virol. 2019. PMID: 30760576 Free PMC article.
-
Clinical Potential of Prefusion RSV F-specific Antibodies.Trends Microbiol. 2018 Mar;26(3):209-219. doi: 10.1016/j.tim.2017.09.009. Epub 2017 Oct 17. Trends Microbiol. 2018. PMID: 29054341 Review.
-
Novel antigens for RSV vaccines.Curr Opin Immunol. 2015 Aug;35:30-8. doi: 10.1016/j.coi.2015.04.005. Epub 2015 Jun 10. Curr Opin Immunol. 2015. PMID: 26070108 Free PMC article. Review.
Cited by
-
Bovine IgG Prevents Experimental Infection With RSV and Facilitates Human T Cell Responses to RSV.Front Immunol. 2020 Aug 6;11:1701. doi: 10.3389/fimmu.2020.01701. eCollection 2020. Front Immunol. 2020. PMID: 32849597 Free PMC article.
-
ChSeq: A database of chameleon sequences.Protein Sci. 2015 Jul;24(7):1075-86. doi: 10.1002/pro.2689. Epub 2015 Jun 16. Protein Sci. 2015. PMID: 25970262 Free PMC article. Review.
-
Computational epitope mapping of class I fusion proteins using low complexity supervised learning methods.PLoS Comput Biol. 2022 Dec 7;18(12):e1010230. doi: 10.1371/journal.pcbi.1010230. eCollection 2022 Dec. PLoS Comput Biol. 2022. PMID: 36477260 Free PMC article.
-
Fusion activation through attachment protein stalk domains indicates a conserved core mechanism of paramyxovirus entry into cells.J Virol. 2014 Apr;88(8):3925-41. doi: 10.1128/JVI.03741-13. Epub 2014 Jan 22. J Virol. 2014. PMID: 24453369 Free PMC article.
-
A protective and safe intranasal RSV vaccine based on a recombinant prefusion-like form of the F protein bound to bacterium-like particles.PLoS One. 2013 Aug 12;8(8):e71072. doi: 10.1371/journal.pone.0071072. eCollection 2013. PLoS One. 2013. PMID: 23951084 Free PMC article.
References
-
- Hall CB. Respiratory syncytial virus. In: Mandell GL, Bennett JE, Dolin R, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 7th Ed. New York: Churchill Livingstone; 2010. pp. 2207–2221.
-
- Johnson S, et al. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J Infect Dis. 1997;176:1215–1224. - PubMed
-
- Kim HW, et al. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am J Epidemiol. 1969;89:422–434. - PubMed
-
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine. 2003;21:3465–3467. - PubMed
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

