The identification and characterization of zebrafish hematopoietic stem cells
- PMID: 21586750
- PMCID: PMC3138684
- DOI: 10.1182/blood-2010-12-327403
The identification and characterization of zebrafish hematopoietic stem cells
Abstract
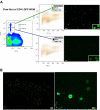
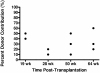
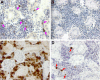
HSCs are defined by their ability to self-renew and maintain hematopoiesis throughout the lifespan of an organism. The optical clarity of their embryos and the ease of genetic manipulation make the zebrafish (Danio rerio) an excellent model for studying hematopoiesis. Using flow cytometry, we identified 2 populations of CD41-GFP(+) cells (GFP(hi) and GFP(lo)) in the whole kidney marrow of Tg(CD41:GFP) zebrafish. Past studies in humans and mice have shown that CD41 is transiently expressed in the earliest hematopoietic progenitors and is then silenced, reappearing in the platelet/thrombocyte lineage. We have transplanted flow-sorted GFP(hi) and GFP(lo) cells into irradiated adult zebrafish and assessed long-term hematopoietic engraftment. Transplantation of GFP(hi) cells did not reconstitute hematopoiesis. In contrast, we observed multilineage hematopoiesis up to 68 weeks after primary and secondary transplantation of GFP(lo) cells. We detected the CD41-GFP transgene in all major hematopoietic lineages and CD41-GFP(+) cells in histologic sections of kidneys from transplant recipients. These studies show that CD41-GFP(lo) cells fulfill generally accepted criteria for HSCs. The identification of fluorescent zebrafish HSCs, coupled with our ability to transplant them into irradiated adult recipients, provide a valuable new tool to track HSC homing, proliferation, and differentiation into hematopoietic cells.
Figures

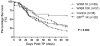

References
-
- McCulloch EA, Till JE. The sensitivity of cells from normal mouse bone marrow to gamma radiation in vitro and in vivo. Radiat Res. 1962;16:822–832. - PubMed
-
- Till JE, Mc CE. A direct measurement of the radiation sensitivity of normal mouse bone marrow cells. Radiat Res. 1961;14:213–222. - PubMed
-
- McCulloch EA, Till JE. The radiation sensitivity of normal mouse bone marrow cells, determined by quantitative marrow transplantation into irradiated mice. Radiat Res. 1960;13:115–125. - PubMed
-
- Weissman IL, Heimfeld S, Spangrude G. Haemopoietic stem cell purification. Immunol Today. 1989;10(6):184–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

