Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration
- PMID: 21586838
- PMCID: PMC3120237
- DOI: 10.4103/0301-4738.81023
Comparative role of intravitreal ranibizumab versus bevacizumab in choroidal neovascular membrane in age-related macular degeneration
Abstract
Context: Ranibizumab and bevacizumab are used widely for treating patients with choroidal neovascular membrane (CNVM) secondary to age-related macular degeneration (AMD).
Aims: To determine and compare the efficacy and safety of intravitreal ranibizumab and bevacizumab in treatment of CNVM due to AMD.
Settings and design: Prospective comparative case series carried out in an eye institute and eye department of a hospital in Kolkata, India.
Materials and methods: One hundred and four eyes with CNVM due to AMD were randomized into two groups. Group A (n=54; 24 occult) received monthly intravitreal ranibizumab injections (0.5 mg in 0.05 ml) and Group B (n=50; 22 occult) received monthly bevacizumab injections (1.25 mg in 0.05 ml) for 3 consecutive months and then as per study criteria. Data analysis done using SPSS software. P-value of <0.05 was considered statistically significant.
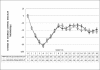
Results: The mean best corrected visual acuity (BCVA) in the ranibizumab group increased from 58.19 Early Treatment Diabetic Retinopathy Study (ETDRS) letters at baseline to 64 ETDRS letters at month 3 (P<0.001). In bevacizumab group mean BCVA increased from 56.80 to 61.72 ETDRS letters at month 3 (P<0.001). At the end of 18 months, there was no statistically significant difference between groups A and B with respect to change in BCVA (P=0.563) or central macular thickness (CMT; P=0.281), as measured by optical coherence tomography (Stratus OCT 3000). No significant sight-threatening complications developed.
Conclusions: Ranibizumab and bevacizumab are equally safe and efficacious in treating CNVM due to AMD.
Conflict of interest statement
Figures

References
-
- Kvanta A, Algvere PV, Berglin L, Seregard S. Subfoveal fibrovascular membranes in age-related macular degeneration express vascular endothelial growth factor. Invest Ophthalmol Vis Sci. 1996;37:1929–34. - PubMed
-
- Otani A, Takagi H, Oh H, Koyama S, Ogura Y, Matumura M, et al. Vascular endothelial growth factor family and receptor expression in human choroidal neovascular membranes. Microvasc Res. 2002;64:162–9. - PubMed
-
- Zarbin M, Szirth B. Current Treatment of Age-Related Macular Degeneration. Optom Vis Sci. 2007;84:559–72. - PubMed
-
- Michels S, Rosenfeld PJ, Puliafito CA, Marcus EN, Venkatraman AS. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration twelve-week results of an uncontrolled open-label clinical study. Ophthalmology. 2005;112:1035–47. - PubMed
-
- First-line treatment of metastatic colorectal cancer [Internet]. FDA Approval for Bevacizumab – National Cancer Institute. [Last cited on 2010 Sep 18]. Available from: http://www.cancer.gov/cancertopics/druginfo/fdabevacizumab#Anchor-Approv... .
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

