Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery
- PMID: 21587211
- PMCID: PMC3182369
- DOI: 10.1038/mt.2011.93
Image-guided, tumor stroma-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated NIS gene delivery
Abstract
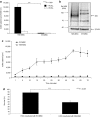
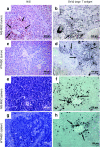
Due to its dual role as reporter and therapy gene, the sodium iodide symporter (NIS) allows noninvasive imaging of functional NIS expression by (123)I-scintigraphy or (124)I-PET imaging before the application of a therapeutic dose of (131)I. NIS expression provides a novel mechanism for the evaluation of mesenchymal stem cells (MSCs) as gene delivery vehicles for tumor therapy. In the current study, we stably transfected bone marrow-derived CD34(-) MSCs with NIS cDNA (NIS-MSC), which revealed high levels of functional NIS protein expression. In mixed populations of NIS-MSCs and hepatocellular cancer (HCC) cells, clonogenic assays showed a 55% reduction of HCC cell survival after (131)I application. We then investigated body distribution of NIS-MSCs by (123)I-scintigraphy and (124)I-PET imaging following intravenous (i.v.) injection of NIS-MSCs in a HCC xenograft mouse model demonstrating active MSC recruitment into the tumor stroma which was confirmed by immunohistochemistry and ex vivo γ-counter analysis. Three cycles of systemic MSC-mediated NIS gene delivery followed by (131)I application resulted in a significant delay in tumor growth. Our results demonstrate tumor-specific accumulation and therapeutic efficacy of radioiodine after MSC-mediated NIS gene delivery in HCC tumors, opening the prospect of NIS-mediated radionuclide therapy of metastatic cancer using MSCs as gene delivery vehicles.
Figures

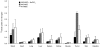


Similar articles
-
Stromal targeting of sodium iodide symporter using mesenchymal stem cells allows enhanced imaging and therapy of hepatocellular carcinoma.Hum Gene Ther. 2013 Mar;24(3):306-16. doi: 10.1089/hum.2012.104. Hum Gene Ther. 2013. PMID: 23402366 Free PMC article.
-
TGFB1-driven mesenchymal stem cell-mediated NIS gene transfer.Endocr Relat Cancer. 2019 Jan 1;26(1):89-101. doi: 10.1530/ERC-18-0173. Endocr Relat Cancer. 2019. PMID: 30121623
-
Mesenchymal stem cell-mediated, tumor stroma-targeted radioiodine therapy of metastatic colon cancer using the sodium iodide symporter as theranostic gene.J Nucl Med. 2015 Apr;56(4):600-6. doi: 10.2967/jnumed.114.146662. Epub 2015 Mar 5. J Nucl Med. 2015. PMID: 25745085
-
A transporter gene (sodium iodide symporter) for dual purposes in gene therapy: imaging and therapy.Curr Gene Ther. 2002 Dec;2(4):393-402. doi: 10.2174/1566523023347599. Curr Gene Ther. 2002. PMID: 12477251 Review.
-
The sodium iodide symporter (NIS): novel applications for radionuclide imaging and treatment.Endocr Relat Cancer. 2021 Sep 3;28(10):T193-T213. doi: 10.1530/ERC-21-0177. Endocr Relat Cancer. 2021. PMID: 34259647 Review.
Cited by
-
Hypoxia-targeted 131I therapy of hepatocellular cancer after systemic mesenchymal stem cell-mediated sodium iodide symporter gene delivery.Oncotarget. 2016 Aug 23;7(34):54795-54810. doi: 10.18632/oncotarget.10758. Oncotarget. 2016. PMID: 27458162 Free PMC article.
-
Imaging and targeted therapy of pancreatic ductal adenocarcinoma using the theranostic sodium iodide symporter (NIS) gene.Oncotarget. 2017 May 16;8(20):33393-33404. doi: 10.18632/oncotarget.16499. Oncotarget. 2017. PMID: 28380420 Free PMC article.
-
The sodium iodide symporter (NIS): regulation and approaches to targeting for cancer therapeutics.Pharmacol Ther. 2012 Sep;135(3):355-70. doi: 10.1016/j.pharmthera.2012.06.007. Epub 2012 Jun 29. Pharmacol Ther. 2012. PMID: 22750642 Free PMC article. Review.
-
Stromal targeting of sodium iodide symporter using mesenchymal stem cells allows enhanced imaging and therapy of hepatocellular carcinoma.Hum Gene Ther. 2013 Mar;24(3):306-16. doi: 10.1089/hum.2012.104. Hum Gene Ther. 2013. PMID: 23402366 Free PMC article.
-
Inflammatory Chemokines MIP-1δ and MIP-3α Are Involved in the Migration of Multipotent Mesenchymal Stromal Cells Induced by Hepatoma Cells.Stem Cells Dev. 2015 May 15;24(10):1223-35. doi: 10.1089/scd.2014.0176. Epub 2015 Mar 3. Stem Cells Dev. 2015. PMID: 25579056 Free PMC article.
References
-
- Fritz V., and, Jorgensen C. Mesenchymal stem cells: an emerging tool for cancer targeting and therapy. Curr Stem Cell Res Ther. 2008;3:32–42. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

