The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans
- PMID: 21587232
- PMCID: PMC3098610
- DOI: 10.1038/ncomms1308
The neural circuits and sensory channels mediating harsh touch sensation in Caenorhabditis elegans
Abstract
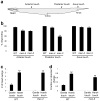
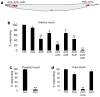
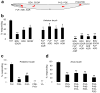
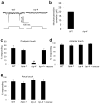
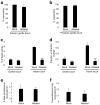
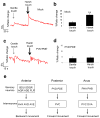
Most animals can distinguish two distinct types of touch stimuli: gentle (innocuous) and harsh (noxious/painful) touch, however, the underlying mechanisms are not well understood. Caenorhabditis elegans is a useful model for the study of gentle touch sensation. However, little is known about harsh touch sensation in this organism. Here we characterize harsh touch sensation in C. elegans. We show that C. elegans exhibits differential behavioural responses to harsh touch and gentle touch. Laser ablations identify distinct sets of sensory neurons and interneurons required for harsh touch sensation at different body segments. Optogenetic stimulation of the circuitry can drive behaviour. Patch-clamp recordings reveal that TRP family and amiloride-sensitive Na(+) channels mediate touch-evoked currents in different sensory neurons. Our work identifies the neural circuits and characterizes the sensory channels mediating harsh touch sensation in C. elegans, establishing it as a genetic model for studying this sensory modality.
Conflict of interest statement
Figures


References
-
- Bounoutas A, Chalfie M. Touch sensitivity in Caenorhabditis elegans. Pflugers Arch. 2007;454:691–702. - PubMed
-
- Purves D, et al. Sensation and sensory processsing. In: Fitzpatrick D, editor. Neuroscience. Sinauer Associates, Inc; Sunderland: 2008. pp. 207–393.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

