4-Methyl-5-phenyl-1H-pyrazol-3-ol
- PMID: 21587918
- PMCID: PMC3006807
- DOI: 10.1107/S1600536810022828
4-Methyl-5-phenyl-1H-pyrazol-3-ol
Abstract
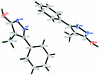
The title compound, C(10)H(10)N(2)O, crystallizes with two independent mol-ecules in the asymmetric unit, having closely comparable geometries. The dihedral angles between the 1H-pyrazole and benzene rings in the two mol-ecules are 39.57 (14) and 41.95 (13)°. The two mol-ecules are each connected to neighbouring mol-ecules by pairs of inter-molecular O-H⋯N hydrogen bonds, forming dimers with R(2) (2)(8) ring motifs. These dimers are further linked into R(4) (4)(10) ring motifs by inter-molecular N-H⋯O hydrogen bonds, forming chains along [101]. The crystal structure is further stabilized by a C-H⋯π inter-action.
Figures
Similar articles
-
5-Pentyl-4-phenyl-sulfonyl-1H-pyrazol-3-ol.Acta Crystallogr Sect E Struct Rep Online. 2010 May 29;66(Pt 6):o1482-3. doi: 10.1107/S1600536810019458. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21579547 Free PMC article.
-
4-[(5-Hydr-oxy-3-methyl-1-phenyl-1H-pyrazol-4-yl)phenyl-meth-yl]-5-methyl-2-phenyl-1H-pyrazol-3(2H)-one ethanol hemisolvate.Acta Crystallogr Sect E Struct Rep Online. 2008 Dec 10;65(Pt 1):o66-7. doi: 10.1107/S1600536808039081. Acta Crystallogr Sect E Struct Rep Online. 2008. PMID: 21581706 Free PMC article.
-
Crystal structure and Hirshfeld surface analysis of N-{2-[(E)-(4-methyl-benzyl-idene)amino]-phen-yl}-2-(5-methyl-1-H-pyrazol-3-yl)acetamide hemihydrate.Acta Crystallogr E Crystallogr Commun. 2019 Jan 8;75(Pt 2):154-158. doi: 10.1107/S2056989018017747. eCollection 2019 Feb 1. Acta Crystallogr E Crystallogr Commun. 2019. PMID: 30800442 Free PMC article.
-
N-(2-Methyl-phen-yl)-6-(1H-pyrazol-1-yl)pyridazin-3-amine.Acta Crystallogr Sect E Struct Rep Online. 2009 Jun 20;65(Pt 7):o1628. doi: 10.1107/S1600536809022867. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21582895 Free PMC article.
-
5-Methoxy-methyl-4-phen-oxy-1H-pyrazol-3-ol.Acta Crystallogr Sect E Struct Rep Online. 2009 Nov 28;65(Pt 12):o3249-50. doi: 10.1107/S1600536809050302. Acta Crystallogr Sect E Struct Rep Online. 2009. PMID: 21578948 Free PMC article.
Cited by
-
β-Keto esters from ketones and ethyl chloroformate: a rapid, general, efficient synthesis of pyrazolones and their antimicrobial, in silico and in vitro cytotoxicity studies.Org Med Chem Lett. 2013 Jul 19;3(1):6. doi: 10.1186/2191-2858-3-6. Org Med Chem Lett. 2013. PMID: 23870758 Free PMC article.
-
3-Ethyl-4-phen-oxy-1-(2,2,2-trifluoro-eth-yl)-1H-pyrazol-5-ol.Acta Crystallogr Sect E Struct Rep Online. 2010 Jul 7;66(Pt 8):o1937-8. doi: 10.1107/S1600536810025948. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21588264 Free PMC article.
-
4-Methyl-5-phenyl-1H-pyrazol-3(2H)-one.Acta Crystallogr Sect E Struct Rep Online. 2010 Dec 18;67(Pt 1):o151-2. doi: 10.1107/S160053681005213X. Acta Crystallogr Sect E Struct Rep Online. 2010. PMID: 21522659 Free PMC article.
References
-
- Allen, F. H., Kennard, O., Watson, D. G., Brammer, L., Orpen, A. G. & Taylor, R. (1987). J. Chem. Soc. Perkin Trans. 2, pp. S1–19.
-
- Bernstein, J., Davis, R. E., Shimoni, L. & Chang, N.-L. (1995). Angew. Chem. Int. Ed. Engl.34, 1555–1573.
-
- Brogden, R. N. (1986). Pyrazolone Derivatives Drugs, 32, 60–70. - PubMed
-
- Bruker (2009). APEX2, SAINT and SADABS. Bruker AXS Inc., Madison, Wiscosin, USA.
-
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
LinkOut - more resources
Full Text Sources
Molecular Biology Databases