Triethyl-ammonium O-3β-cholest-5-en-3-yl (4-meth-oxy-phen-yl)dithio-phospho-nate
- PMID: 21588433
- PMCID: PMC3007563
- DOI: 10.1107/S1600536810029703
Triethyl-ammonium O-3β-cholest-5-en-3-yl (4-meth-oxy-phen-yl)dithio-phospho-nate
Abstract
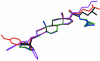
In the crystal structure of the title compound, C(6)H(16)N(+)·C(34)H(52)O(2)PS(2) (-) or [(CH(3)CH(2))(3)NH](+)·[C(34)H(52)O(2)PS(2)](-), the cation and anion are paired via weak, inter-molecular, bifurcated N-H⋯(S,S) hydrogen bonds. The cholesteryl units form an alternating (herringbone) motif as well as an infinitely stacked layered structure along the b axis. The P-S bond lengths [1.975 (2) and 1.981 (2) Å compared with ca 1.92 Å for a formal P=S double bond and with ca 2.01 Å for a P-S single bond] suggest delocalization of the negative charge between the P-S bonds. A distorted tetra-hedral geometry around the P atom is revealed by non-ideal O-P-C and S-P-S bond angles of 96.7 (2) and 115.52 (11)°, respectively.
Figures

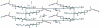
References
-
- Beaton, G., Dellinger, D., Marshall, W. S. & Caruthers, M. H. (1991). Oligonucleotides analogues, edited by F. Eckstein, pp. 109–135.
-
- Blaszczyk, J., Wieczorek, M. W., Okruszek, A., Sierzchala, A., Kobylanska, A. & Stec, W. J. (1996). J. Chem. Cryst.26, 33–42.
-
- Brandenburg, K. & Brendt, M. (2001). DIAMOND Crystal Impact GbR, Postfach 1251, D-53002 Bonn, Germany.
-
- Bromberg, L., Lewin, I. & Warshawsky, A. (1993). Hydrometallurgy, 33, 59–71.
-
- Bruker (1998). SADABS and SMART-NT Bruker AXS Inc., Madison, Wisconsin, USA.
LinkOut - more resources
Full Text Sources
Research Materials
