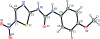
N-(4-Meth-oxy-phen-yl)-N'-(5-nitro-1,3-thia-zol-2-yl)urea
- PMID: 21588684
- PMCID: PMC3008116
- DOI: 10.1107/S1600536810032186
N-(4-Meth-oxy-phen-yl)-N'-(5-nitro-1,3-thia-zol-2-yl)urea
Abstract
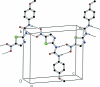
The title compound, C(11)H(10)N(4)O(4)S, is a derivative of N-(4-meth-oxy-benz-yl)-N'-(5-nitro-1,3-thia-zol-2-yl)urea (AR-A014418), a known glycogen synthase kinase 3β (GSK-3β) inhibitor. All non-H atoms in the mol-ecule are essentially coplanar, with an r.m.s. deviation of 0.045 Å and a maximum deviation of 0.115 (2) Å for the carbonyl O atom. In the crystal structure, mol-ecules are linked via N-H⋯O hydrogen bonds into one-dimensional chains along [101].
Figures
References
-
- Altomare, A., Cascarano, G., Giacovazzo, C., Guagliardi, A., Burla, M. C., Polidori, G. & Camalli, M. (1994). J. Appl. Cryst.27, 435.
-
- Bhat, R., Xue, Y. F., Berg, S., Hellberg, S., Ormo, M., Nilsson, Y., Radesater, A. C., Jerning, E., Markgren, P. O., Borgegard, T., Nylof, M., Gimenez-Cassina, A., Hernandez, F., Lucas, J. J., Diaz-Nido, J. & Avila, J. (2003). J. Biol. Chem.278, 45937–45945. - PubMed
-
- Blessing, R. H. (1995). Acta Cryst. A51, 33–38. - PubMed
-
- Inestrosa, N. C., Farías, G. & Colombres, M. (2006). Glycogen Synthase Kinase 3 (GSK-3) and Its Inhibitors: Drug Discovery and Development, edited by A. Martinez, A. Castro & M. Medina, pp. 25–43. New Jersey: John Wiley & Sons Inc.
-
- Nonius (2002). COLLECT Nonius BV, Delft, The Netherlands.
LinkOut - more resources
Full Text Sources
Research Materials