3-Methyl-4-[(E)-3-thien-ylmethyl-idene-amino]-1H-1,2,4-triazole-5(4H)-thione
- PMID: 21589044
- PMCID: PMC3009062
- DOI: 10.1107/S1600536810041152
3-Methyl-4-[(E)-3-thien-ylmethyl-idene-amino]-1H-1,2,4-triazole-5(4H)-thione
Abstract
The asymmetric unit of the title compound, C(8)H(8)N(4)S(2), contains two crystallographically independent mol-ecules. The thio-phene ring of one mol-ecule is disordered over two positions with refined site occupancies of 0.6375 (19) and 0.3625 (19). One mol-ecule is almost planar and the other one is twisted, the dihedral angles between the thio-phene and triazole rings being 7.28 (7) and 48.9 (2)° [48.5 (4)° for the minor component], respectively. An intra-molecular C-H⋯S hydrogen bond stabilizes the mol-ecular conformation of the planar molecule. In the crystal, the two mol-ecules are inter-connected by N-H⋯S hydrogen bonds into dimers, which are further consolidated into chains along the b axis by C-H⋯N hydrogen bonds. Weak C-H⋯π and π-π inter-actions [centroid-centroid distance = 3.5149 (7) Å] are also observed.
Figures

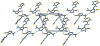
References
-
- Aytac, S. P., Tozkoparan, B., Kaynak, F. B., Aktay, G., Goktas, O. & Unuvar, S. (2009). Eur. J. Med. Chem.44, 4528–4538. - PubMed
-
- Bruker (2009). APEX2, SAINT and SADABS Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Cosier, J. & Glazer, A. M. (1986). J. Appl. Cryst.19, 105–107.
-
- Ghazzali, M., Al-Farhan, K., El-Faham, A. & Reedijk, J. (2010). Polyhedron, 29, 2829–2832.
-
- Kucukguzel, I., Tatar, E., Kucukguzel, S. G., Rolollas, S. & Clercq, E. D. (2008). Eur. J. Med. Chem.43, 381–392. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
