6-[Bis(ethoxycarbonyl)methyl]-6-deoxy-1,2;3,4-di-O-isopropyl-idene-d-galacto-pyran-ose
- PMID: 21589509
- PMCID: PMC3011695
- DOI: 10.1107/S1600536810046921
6-[Bis(ethoxycarbonyl)methyl]-6-deoxy-1,2;3,4-di-O-isopropyl-idene-d-galacto-pyran-ose
Abstract
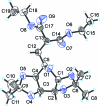
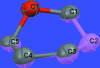
The title compound, C(19)H(30)O(9), was prepared by substitution at the C6 position in 1,2;3,4-di-O-isopropyl-idene-6-O-trifluoro-methane-sulfonyl-d-galactose using sodium eth-oxy-malonate in dimethyl-formamide. The conformation is skew-boat (0)S(2), slightly distorted towards boat B(2,5). The inflexible pyran-ose structure makes the title compound a suitable inter-mediate for further synthetic work by keeping stereogenic carbon atoms safe from inversion. Several short intra-molecular C-H⋯ O contacts may stabilize the conformation of the mol-ecule. Inter-molecular C-H⋯O inter-actions also occur.
Figures



References
-
- Berces, A., Whitfield, D. M. & Nukada, T. (2001). Tetrahedron, 57, 477–491.
-
- Boeyens, J. C. A. (1978). J. Cryst. Mol. Struct.8, 317–320.
-
- Boeyens, J. C. A., Rathbone, E. B. & Woolard, G. R. (1978). Carbohydr. Res.62, 39–47.
-
- Bouhlal, D., Martin, P., Massoui, M., Nowogrocki, G., Pilard, S., Villa, P. & Goethals, G. (2001). Tetrahedron Asymmetry, 12, 1573–1577.
-
- Cipolla, L., Liguori, L., Nicotra, F., Torri, G. & Vismara, E. (1996). Chem. Commun., pp. 1253–1254.
LinkOut - more resources
Full Text Sources
Miscellaneous
