(2S)-2-[(2S*,5R*,6R*)-5,6-Dimeth-oxy-5,6-dimethyl-1,4-dioxan-2-yl]-1-[(S)-1,1-dimethyl-ethylsulfon-yl]aziridine
- PMID: 21589609
- PMCID: PMC3011582
- DOI: 10.1107/S1600536810048816
(2S)-2-[(2S*,5R*,6R*)-5,6-Dimeth-oxy-5,6-dimethyl-1,4-dioxan-2-yl]-1-[(S)-1,1-dimethyl-ethylsulfon-yl]aziridine
Abstract
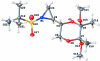
The reaction of a sulfur ylide with a chiral non-racemic sulfinyl imine afforded the desired aziridine in excellent yield and subsequent oxidation of the sulfinyl moiety dissolved in anhydrous dichloro-methane using a 75% aqueous solution of 3-chloro-per-oxy-benzoic acid afforded the title compound, C(14)H(27)NO(6)S. The configuration of the newly formed stereogenic center at the point of attachment of the 1,4-dioxane ring to the aziridine ring is S. The configurations of the pre-existing sites 2-, 5-, and 6-positions of the 1,4-dioxane ring prior to reaction of sulfinyl imine with the sulfur ylide are S, R, and R, respectively. The C-N bond lengths of the aziridine are 1.478 (2) and 1.486 (2) Å.
Figures
References
-
- Chigboh, K., Morton, D., Nadin, A. & Stockman, R. A. (2008). Tetrahedron Lett.49, 4768–4770.
-
- Clark, R. C. & Reid, J. S. (1995). Acta Cryst. A51, 887–897.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst.42, 339–341.
-
- Ellman, J. A., Owens, T. D. & Tang, P. T. (2002). Acc. Chem. Res.35, 984–995. - PubMed
-
- Flack, H. D. (1983). Acta Cryst. A39, 876–881.
Grants and funding
LinkOut - more resources
Full Text Sources