Application of hydroxyapatite nanoparticles in development of an enhanced formulation for delivering sustained release of triamcinolone acetonide
- PMID: 21589650
- PMCID: PMC3090279
- DOI: 10.2147/IJN.S18045
Application of hydroxyapatite nanoparticles in development of an enhanced formulation for delivering sustained release of triamcinolone acetonide
Abstract
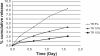
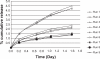
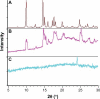
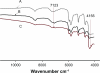
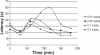
We report an analysis of in vitro and in vivo drug release from an in situ formulation consisting of triamcinolone acetonide (TR) and poly(D,L-lactide-co-glycolide) (PLGA) and the additives glycofurol (GL) and hydroxyapatite nanoparticles (HA). We found that these additives enhanced drug release rate. We used the Taguchi method to predict optimum formulation variables to minimize the initial burst. This method decreased the burst rate from 8% to 1.3%. PLGA-HA acted as a strong buffer, thereby preventing tissue inflammation at the injection site caused by the acidic degradation products of PLGA. Characterization of the optimized formulation by a variety of techniques, including scanning electron microscopy, X-ray diffraction, differential scanning calorimetry, and Fourier transform near infrared spectroscopy, revealed that the crystalline structure of TR was converted to an amorphous form. Therefore, this hydrophobic agent can serve as an additive to modify drug release rates. Data generated by in vitro and in vivo experiments were in good agreement.
Keywords: PLGA; glycofurol; hydroxyapatite nanoparticle; triamcinolone acetonide.
Figures







References
-
- Kakinoki S, Taguchi T. Antitumor effect of an injectable in-situ forming drug delivery system composed of a novel tissue adhesive containing doxorubicin hydrochloride. Eur J Pharm Biopharm. 2007;67:676–681. - PubMed
-
- Lee F, Chung JE, Kurisawa M. An injectable hyaluronic acid-tyramine hydrogel system for protein delivery. J Control Release. 2009;134:186–193. - PubMed
-
- Fraylich MR, Liu R, Richardson SM, et al. Thermally-triggered gelation of PLGA dispersions: towards an injectable colloidal cell delivery system. J Colloid Interface Sci. 2010;344:61–69. - PubMed
-
- Hollister LE. Site-specific drug delivery to CNS: Old and new. Neurobiol Aging. 1989;10:628–631. - PubMed
-
- Levy RJ, Vinod L, Strickberger SA, Underwood T, Davis J. Controlled release implant dosage forms for cardiac arrhythmias: review and perspectives. Drug Delivery. 1996;3:137–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

