Cancer screening by systemic administration of a gene delivery vector encoding tumor-selective secretable biomarker expression
- PMID: 21589655
- PMCID: PMC3092745
- DOI: 10.1371/journal.pone.0019530
Cancer screening by systemic administration of a gene delivery vector encoding tumor-selective secretable biomarker expression
Abstract
Cancer biomarkers facilitate screening and early detection but are known for only a few cancer types. We demonstrated the principle of inducing tumors to secrete a serum biomarker using a systemically administered gene delivery vector that targets tumors for selective expression of an engineered cassette. We exploited tumor-selective replication of a conditionally replicative Herpes simplex virus (HSV) combined with a replication-dependent late viral promoter to achieve tumor-selective biomarker expression as an example gene delivery vector. Virus replication, cytotoxicity and biomarker production were low in quiescent normal human foreskin keratinocytes and high in cancer cells in vitro. Following intravenous injection of virus >90% of tumor-bearing mice exhibited higher levels of biomarker than non-tumor-bearing mice and upon necropsy, we detected virus exclusively in tumors. Our strategy of forcing tumors to secrete a serum biomarker could be useful for cancer screening in high-risk patients, and possibly for monitoring response to therapy. In addition, because oncolytic vectors for tumor specific gene delivery are cytotoxic, they may supplement our screening strategy as a "theragnostic" agent. The cancer screening approach presented in this work introduces a paradigm shift in the utility of gene delivery which we foresee being improved by alternative vectors targeting gene delivery and expression to tumors. Refining this approach will usher a new era for clinical cancer screening that may be implemented in the developed and undeveloped world.
Conflict of interest statement
Figures


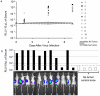
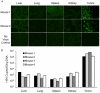
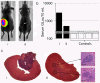
References
-
- Maeda H. The enhanced permeability and retention (EPR) effect in tumor vasculature: the key role of tumor-selective macromolecular drug targeting. Advan Enzyme Regul. 2001;41:189–207. - PubMed
-
- Maeda H, Fanga J, Inutsuka T, Kitamotoc Y. Vascular permeability enhancement in solid tumor: various factors, mechanisms involved and its implications. International Immunopharmacology. 2003;3:319–328. - PubMed
-
- Kirpotin DB, Drummond DarylC, Shao Yi, Shalaby M Refaat, Hong Keelung, Nielsen UlrikB, Marks JamesD, Benz ChristopherC, Park JohnW. Antibody Targeting of Long-Circulating Lipidic Nanoparticles Does Not Increase Tumor Localization but Does Increase Internalization in Animal Models. Cancer Res. 2006;66:6732–6740. - PubMed
-
- Kocbek P, Obermajer N, Cegnar M, Kos J, Kristl J. Targeting cancer cells using PLGA nanoparticles surface modified with monoclonal antibody. Journal of Controlled Release. 2007;120:18–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

