Niosome as a drug carrier for topical delivery of N-acetyl glucosamine
- PMID: 21589799
- PMCID: PMC3093622
Niosome as a drug carrier for topical delivery of N-acetyl glucosamine
Abstract
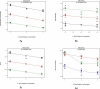
Niosomes are non-ionic surfactant vesicles that have potential applications in the delivery of hydrophilic and hydrophobic drugs. The topical form of N-acetyl glucosamine (NAG) recently has been considered in the treatment of hyperpigmentation disorders due to its inhibitory effect on thyrosinase enzymes in melanocytes. To improve NAG penetration into the skin we formulated the drug in niosomes and investigated its flux across excised rat skin using Franz diffusion cells. The drug assay was performed by a novel and specific high performance liquid chromatography method. Niosomal vesicles were further characterized by optical and scanning electron microscopy and particle size analysis. Niosomes prepared with Span 40 produced a drug encapsulation of about 50%. The vesicle size was markedly dependent on the composition of the niosome formulations and was in range of 500-4500 nm (Span 80 < Span 60 < Span 40 niosomes). Span 40-niosomes provided a higher NAG flux across the skin than Span 60- and Span 80-nisomes. All formulations significantly improved the extent of drug assessed to be localized in the skin (P< 0.05), as compared to NAG hydroalcoholic (HA) solution. Our study demonstrated the potential of niosomes for improved NAG localization in the skin, as needed in hyperpigmentation disorders.
Keywords: HPLC; N-Acetyl Glucosamine; Noisome; Topical delivery.
Figures









References
-
- Couvreur P, Fattal E, Andremont A. Liposomes and nanoparticles in the treatment of intracellular bacterial infections. Pharm Res. 1991;8:1079–1086. - PubMed
-
- Schreier H, Bouwstra J. Liposomes and niosomes as topical drug carriers: dermal and transdermal drug delivery. J Control Rel. 1994;30:1–15.
-
- Uchegbu IF, Vyas SP. Non-Ionic surfactant based vesicles (niosomes) in drug delivery. Int J Pharm. 1998;172:33–70.
-
- Bandak S, Ramu A, Barenholz Y. Reduced UV-induced degradation of doxorubicin encapsulated in polyethyleneglycol-coated leptosomes. Pham Res. 1999;16:841–846. - PubMed
-
- Tsuchihashi M, Harashima H, Kiwada H. Development of pharmacokinetic / pharmacodynamic (PK/PD)-simulation system for doxorubicin in long circulating leptosomes in mice using peritoneal. J control Rel. 1999;61:9–19. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
