Quantitative subcellular proteome and secretome profiling of influenza A virus-infected human primary macrophages
- PMID: 21589892
- PMCID: PMC3093355
- DOI: 10.1371/journal.ppat.1001340
Quantitative subcellular proteome and secretome profiling of influenza A virus-infected human primary macrophages
Abstract
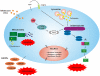
Influenza A viruses are important pathogens that cause acute respiratory diseases and annual epidemics in humans. Macrophages recognize influenza A virus infection with their pattern recognition receptors, and are involved in the activation of proper innate immune response. Here, we have used high-throughput subcellular proteomics combined with bioinformatics to provide a global view of host cellular events that are activated in response to influenza A virus infection in human primary macrophages. We show that viral infection regulates the expression and/or subcellular localization of more than one thousand host proteins at early phases of infection. Our data reveals that there are dramatic changes in mitochondrial and nuclear proteomes in response to infection. We show that a rapid cytoplasmic leakage of lysosomal proteins, including cathepsins, followed by their secretion, contributes to inflammasome activation and apoptosis seen in the infected macrophages. Also, our results demonstrate that P2X₇ receptor and src tyrosine kinase activity are essential for inflammasome activation during influenza A virus infection. Finally, we show that influenza A virus infection is associated with robust secretion of different danger-associated molecular patterns (DAMPs) suggesting an important role for DAMPs in host response to influenza A virus infection. In conclusion, our high-throughput quantitative proteomics study provides important new insight into host-response against influenza A virus infection in human primary macrophages.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124:783–801. - PubMed
-
- Kawai T, Akira S. Innate immune recognition of viral infection. Nat Immunol. 2006;7:131–137. - PubMed
-
- Koyama S, Ishii KJ, Kumar H, Tanimoto T, Coban C, et al. Differential role of TLR- and RLR-signaling in the immune responses to influenza A virus infection and vaccination. J Immunol. 2007;179:4711–4720. - PubMed
-
- Pirhonen J, Sareneva T, Kurimoto M, Julkunen I, Matikainen S. Virus infection activates IL-1β and IL-18 production in human macrophages by a caspase-1-dependent pathway. J Immunol. 1999;162:7322–7329. - PubMed
-
- Pirhonen J, Sareneva T, Julkunen I, Matikainen S. Virus infection induces proteolytic processing of IL-18 in human macrophages via caspase-1 and caspase-3 activation. Eur J Immunol. 2001;31:726–733. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

