Human neutrophil clearance of bacterial pathogens triggers anti-microbial γδ T cell responses in early infection
- PMID: 21589907
- PMCID: PMC3093373
- DOI: 10.1371/journal.ppat.1002040
Human neutrophil clearance of bacterial pathogens triggers anti-microbial γδ T cell responses in early infection
Abstract
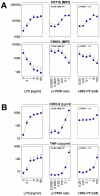
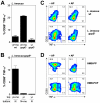
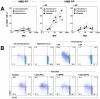
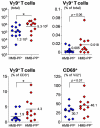
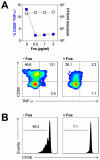
Human blood Vγ9/Vδ2 T cells, monocytes and neutrophils share a responsiveness toward inflammatory chemokines and are rapidly recruited to sites of infection. Studying their interaction in vitro and relating these findings to in vivo observations in patients may therefore provide crucial insight into inflammatory events. Our present data demonstrate that Vγ9/Vδ2 T cells provide potent survival signals resulting in neutrophil activation and the release of the neutrophil chemoattractant CXCL8 (IL-8). In turn, Vγ9/Vδ2 T cells readily respond to neutrophils harboring phagocytosed bacteria, as evidenced by expression of CD69, interferon (IFN)-γ and tumor necrosis factor (TNF)-α. This response is dependent on the ability of these bacteria to produce the microbial metabolite (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate (HMB-PP), requires cell-cell contact of Vγ9/Vδ2 T cells with accessory monocytes through lymphocyte function-associated antigen-1 (LFA-1), and results in a TNF-α dependent proliferation of Vγ9/Vδ2 T cells. The antibiotic fosmidomycin, which targets the HMB-PP biosynthesis pathway, not only has a direct antibacterial effect on most HMB-PP producing bacteria but also possesses rapid anti-inflammatory properties by inhibiting γδ T cell responses in vitro. Patients with acute peritoneal-dialysis (PD)-associated bacterial peritonitis--characterized by an excessive influx of neutrophils and monocytes into the peritoneal cavity--show a selective activation of local Vγ9/Vδ2 T cells by HMB-PP producing but not by HMB-PP deficient bacterial pathogens. The γδ T cell-driven perpetuation of inflammatory responses during acute peritonitis is associated with elevated peritoneal levels of γδ T cells and TNF-α and detrimental clinical outcomes in infections caused by HMB-PP positive microorganisms. Taken together, our findings indicate a direct link between invading pathogens, neutrophils, monocytes and microbe-responsive γδ T cells in early infection and suggest novel diagnostic and therapeutic approaches.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






References
-
- Nathan N. Neutrophils and immunity: challenges and opportunities. Nat Rev Immunol. 2006;6:173–182. - PubMed
-
- Müller I, Munder M, Kropf P, Hänsch GM. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 2009;30:522–530. - PubMed
-
- Weaver CT, Hatton RD, Mangan PR, Harrington LE. IL-17 family cytokines and the expanding diversity of effector T cell lineages. Annu Rev Immunol. 2007;25:821–852. - PubMed
-
- Sallusto F, Lanzavecchia A. Heterogeneity of CD4+ memory T cells: functional modules for tailored immunity. Eur J Immunol. 2009;39:2076–2082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

