Degradation of the disease-associated prion protein by a serine protease from lichens
- PMID: 21589935
- PMCID: PMC3092769
- DOI: 10.1371/journal.pone.0019836
Degradation of the disease-associated prion protein by a serine protease from lichens
Abstract
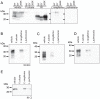
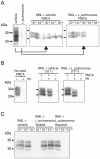
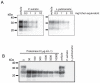
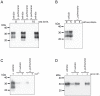
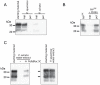
The disease-associated prion protein (PrP(TSE)), the probable etiological agent of the transmissible spongiform encephalopathies (TSEs), is resistant to degradation and can persist in the environment. Lichens, mutualistic symbioses containing fungi, algae, bacteria and occasionally cyanobacteria, are ubiquitous in the environment and have evolved unique biological activities allowing their survival in challenging ecological niches. We investigated PrP(TSE) inactivation by lichens and found acetone extracts of three lichen species (Parmelia sulcata, Cladonia rangiferina and Lobaria pulmonaria) have the ability to degrade prion protein (PrP) from TSE-infected hamsters, mice and deer. Immunoblots measuring PrP levels and protein misfolding cyclic amplification indicated at least two logs of reductions in PrP(TSE). Degradative activity was not found in closely related lichen species or in algae or a cyanobacterium that inhabit lichens. Degradation was blocked by Pefabloc SC, a serine protease inhibitor, but not inhibitors of other proteases or enzymes. Additionally, we found that PrP levels in PrP(TSE)-enriched preps or infected brain homogenates are also reduced following exposure to freshly-collected P. sulcata or an aqueous extract of the lichen. Our findings indicate that these lichen extracts efficiently degrade PrP(TSE) and suggest that some lichens could have potential to inactivate TSE infectivity on the landscape or be a source for agents to degrade prions. Further work to clone and characterize the protease, assess its effect on TSE infectivity and determine which organism or organisms present in lichens produce or influence the protease activity is warranted.
Conflict of interest statement
Figures




References
-
- Taylor DM. Inactivation of transmissible degenerative encephalopathy agents: A review. Vet J. 2000;159:10–17. - PubMed
-
- Brown P, Preece M, Brandel JP, Sato T, McShane L, et al. Iatrogenic Creutzfeldt-Jakob disease at the millennium. Neurology. 2000;55:1075–1081. - PubMed
-
- Hoinville LJ. A review of the epidemiology of scrapie in sheep. Rev Sci Tech. 1996;15:827–852. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

