Complete budding and asymmetric division of primitive model cells to produce daughter vesicles with different interior and membrane compositions
- PMID: 21591721
- PMCID: PMC3115689
- DOI: 10.1021/ja202406v
Complete budding and asymmetric division of primitive model cells to produce daughter vesicles with different interior and membrane compositions
Abstract
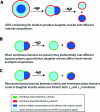
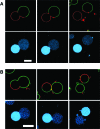
Asymmetric cell division is common in biology and plays critical roles in differentiation and development. Unicellular organisms are often used as model systems for understanding the origins and consequences of asymmetry during cell division. Although basic as compared to mammalian cells, these are already quite complex. We report complete budding and asymmetric fission of very simple nonliving model cells to produce daughter vesicles that are chemically distinct in both interior and membrane compositions. Our model cells are based on giant lipid vesicles (GVs, 10-30 μm) encapsulating a polyethylene glycol (PEG)/dextran aqueous two-phase system (ATPS) as a crowded and compartmentalized cytoplasm mimic. Ternary lipid compositions were used to provide coexisting micrometer-scale liquid disordered (L(d)) and liquid ordered (L(o)) domains in the membranes. ATPS-containing vesicles formed buds when sucrose was added externally to provide increased osmotic pressure, such that they became not only morphologically asymmetric but also asymmetric in both their interior and their membrane compositions. Further increases in osmolality drove formation of two chemically distinct daughter vesicles, which were in some cases connected by a lipid nanotube (complete budding), and in others were not (fission). In all cases, separation occurred at the aqueous-aqueous phase boundary, such that one daughter vesicle contained the PEG-rich aqueous phase and the other contained the dextran-rich aqueous phase. PEGylated lipids localized in the L(o) domain resulted in this membrane domain preferentially coating the PEG-rich bud prior to division, and subsequently the PEG-rich daughter vesicle. Varying the mole ratio of lipids resulted in excess surface area of L(o) or L(d) membrane domains such that, upon division, this excess portion was inherited by one of the daughter vesicles. In some cases, a second "generation" of aqueous phase separation and budding could be induced in these daughter vesicles. Asymmetric fission of a simple self-assembled model cell, with production of daughter vesicles that harbored different protein concentrations and lipid compositions, is an example of the seemingly complex behavior possible for simple molecular assemblies. These compartmentalized and asymmetrically dividing ATPS-containing GVs could serve as a test bed for investigating possible roles for spatial and organizational cues in asymmetric cell division and inheritance.
Figures





References
-
- Menger F. M.; Gabrielson K. D. Angew. Chem., Int. Ed. Engl. 1995, 34, 2091–2106.
- Menger F. M.; Angelova M. I. Acc. Chem. Res. 1998, 31, 789–797.
- Oberholzer T.; Luisi P. L. J. Biol. Phys. 2002, 28, 733–744. - PMC - PubMed
- Liposomes: A Practical Approach, 2nd ed.; Torchilin V. P., Weissig V., Eds.; Oxford University Press: Oxford, 1990.
-
- Giant Vesicles, Perspectives in Supramolecular Chemistry 6; Luisi P. L., Walde, Eds.; John Wiley and Sons: West Sussex, 2000.
-
- Walde P.; Cosentino K.; Engel H.; Stano P. ChemBioChem 2010, 11, 848–865. - PubMed
-
- Jesorka A.; Orwar O. Annu. Rev. Anal. Chem. 2008, 1, 801–832. - PubMed
-
- Dimova R.; Aranda S.; Bezlyepkina N.; Nikolov V.; Riske K. A.; Lipowsky R. J. Phys.: Condens. Matter 2006, 18, S1151–S1176. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

