α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells
- PMID: 21592114
- PMCID: PMC3142646
- DOI: 10.1111/j.1365-2249.2011.04421.x
α-Fetoprotein impairs activation of natural killer cells by inhibiting the function of dendritic cells
Abstract
α-Fetoprotein (AFP) is a tumour-associated antigen in hepatocellular carcinoma (HCC). The biological properties of AFP have been identified in its regulatory effects on immune responses of T cells and B cells. However, AFP effects on natural killer (NK) cells are still unclear. In this study, we examined the immunoregulation of AFP on NK activity. The cytolytic activity against K562 cells and Huh7 cells of NK cells co-cultured with AFP-treated dendritic cells (DCs) (AFP-DCs) was lower than that with albumin-treated DCs (Alb-DCs). Direct addition of AFP to NK cells did not alter the cytolytic activity of NK cells. Adding AFP inhibited the interleukin (IL)-12 production of DCs after stimulation with lipopolysaccharide (LPS) [Toll-like receptor (TLR)-4 ligand], or Poly(I:C) (TLR-3 ligand), but not IL-18 production. The mRNAs of IL-12p35 and IL-12p40 were significantly inhibited in AFP-DCs compared with Alb-DCs, but those of TLR-4 or TLR-3 were not. Transwell experiments revealed that soluble factors derived from DCs played roles in inhibition of the ability of activating NK cells by AFP-DCs. Adding the neutralizing antibody of IL-12 to NK cells co-cultured with Alb-DCs resulted in a decrease of cytolytic activity to the levels of NK cells co-cultured with AFP-DCs. Adding IL-12 to NK cells co-cultured with AFP-DCs resulted in an increase of cytolytic activity to the levels of NK cells co-cultured with Alb-DCs. These demonstrated that the impairment of IL-12 production from AFP-DCs resulted in inhibition of the ability of the activation of NK cells by DCs, and thus suggests a role of AFP in HCC development.
© 2011 The Authors. Clinical and Experimental Immunology © 2011 British Society for Immunology.
Figures

 ) or Alb-DCs (♦) against K562 cells (a) or Huh7 cells (b) were evaluated by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. (c) NK cells were isolated from PBMCs by magnetic cell sorting using CD56 MicroBeads, according to the manufacturer's instructions. Enriched NK cells were co-cultured with AFP (25 µg/ml, AFP-DCs) or Alb (25 µg/ml, Alb-DCs) pretreated DCs for 24 h. The IFN-γ productions from NK cells were analysed by specific enzyme-linked immunosorbent assay (ELISA). To evaluate the IFN-γ production from NK cells, we also evaluated the IFN-γ production from AFP-DCs or Alb-DCs cultured without NK cells, and these values were subtracted from all experimental determinations to determine specific IFN-γ productions of NK cells (results in pg/ml; mean ± standard deviation of triplicate samples). We analysed statistically the production of IFN-γ between AFP-DCs and Alb-DCs. *P < 0·05.
) or Alb-DCs (♦) against K562 cells (a) or Huh7 cells (b) were evaluated by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. (c) NK cells were isolated from PBMCs by magnetic cell sorting using CD56 MicroBeads, according to the manufacturer's instructions. Enriched NK cells were co-cultured with AFP (25 µg/ml, AFP-DCs) or Alb (25 µg/ml, Alb-DCs) pretreated DCs for 24 h. The IFN-γ productions from NK cells were analysed by specific enzyme-linked immunosorbent assay (ELISA). To evaluate the IFN-γ production from NK cells, we also evaluated the IFN-γ production from AFP-DCs or Alb-DCs cultured without NK cells, and these values were subtracted from all experimental determinations to determine specific IFN-γ productions of NK cells (results in pg/ml; mean ± standard deviation of triplicate samples). We analysed statistically the production of IFN-γ between AFP-DCs and Alb-DCs. *P < 0·05.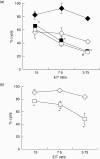
 ) or Alb-DCs (♦) for 24 h. We evaluated the cytolytic activity of AFP-NK cells and Alb-NK cells or NK cells stimulated by AFP-DCs or Alb-DCs using K562 cells as target cells by 51Cr-releasing assay. We analysed statistically between the cytolytic activity of NK cells co-cultured with AFP-DCs (
) or Alb-DCs (♦) for 24 h. We evaluated the cytolytic activity of AFP-NK cells and Alb-NK cells or NK cells stimulated by AFP-DCs or Alb-DCs using K562 cells as target cells by 51Cr-releasing assay. We analysed statistically between the cytolytic activity of NK cells co-cultured with AFP-DCs ( ) and Alb-DCs (♦) or between that of AFP-NK cells (□) and Alb-NK cells (◊). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs, #P < 0·05 versus the cytolytic activity of Alb-NK cells. (b) Enriched NK cells were co-cultured with AFP-DCs (□) or Alb-DCs (◊) for 24 h in the presence of 0·4 µm of inserting membrane (Transwell). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.
) and Alb-DCs (♦) or between that of AFP-NK cells (□) and Alb-NK cells (◊). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs, #P < 0·05 versus the cytolytic activity of Alb-NK cells. (b) Enriched NK cells were co-cultured with AFP-DCs (□) or Alb-DCs (◊) for 24 h in the presence of 0·4 µm of inserting membrane (Transwell). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr-releasing assay. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.


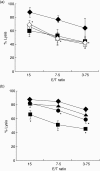
 ) or Alb-DCs (♦) without neutralizing antibody of IL-12 or AFP-DCs (□) or Alb-DCs (◊) with neutralizing antibody of IL-12. We analysed statistically between the antibody-adding and not-adding groups in both AFP-DC and Alb-DC cultures, respectively. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Significant difference was observed between the antibody-adding group and not-adding group in the cytolytic activity of NK cells cultured with Alb-DCs. In contrast, no significant difference was observed between the groups in the cytolytic activity of NK cells cultured with AFP-DCs. (b) Enriched NK cells were co-cultured with AFP-DCs or Alb-DCs for 24 h with or without recombinant IL-12p70 protein (150 pg/ml, 300 pg/ml). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr releasing assay. The cytolytic activity co-cultured with AFP-DCs without IL-12 (
) or Alb-DCs (♦) without neutralizing antibody of IL-12 or AFP-DCs (□) or Alb-DCs (◊) with neutralizing antibody of IL-12. We analysed statistically between the antibody-adding and not-adding groups in both AFP-DC and Alb-DC cultures, respectively. *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Significant difference was observed between the antibody-adding group and not-adding group in the cytolytic activity of NK cells cultured with Alb-DCs. In contrast, no significant difference was observed between the groups in the cytolytic activity of NK cells cultured with AFP-DCs. (b) Enriched NK cells were co-cultured with AFP-DCs or Alb-DCs for 24 h with or without recombinant IL-12p70 protein (150 pg/ml, 300 pg/ml). NK cells were harvested and subjected to examine the cytolytic activity against K562 cells by 51Cr releasing assay. The cytolytic activity co-cultured with AFP-DCs without IL-12 ( ) or with IL-12 (150 pg/ml, •; 300 pg/ml, ▴) or Alb-DCs (♦). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.
) or with IL-12 (150 pg/ml, •; 300 pg/ml, ▴) or Alb-DCs (♦). *P < 0·05 versus the cytolytic activity of NK cells cultured with Alb-DCs. Representative results are shown. Similar results were obtained from three independent experiments in all experiments.Similar articles
-
Patient-derived dendritic cells transduced with an a-fetoprotein-encoding adenovirus and co-cultured with autologous cytokine-induced lymphocytes induce a specific and strong immune response against hepatocellular carcinoma cells.Liver Int. 2006 Apr;26(3):369-79. doi: 10.1111/j.1478-3231.2005.01235.x. Liver Int. 2006. PMID: 16584401
-
alpha-fetoprotein and interleukin-18 gene-modified dendritic cells effectively stimulate specific type-1 CD4- and CD8-mediated T-Cell response from hepatocellular carcinoma patients in Vitro.Hum Immunol. 2007 May;68(5):334-41. doi: 10.1016/j.humimm.2007.01.008. Epub 2007 Feb 21. Hum Immunol. 2007. PMID: 17462500
-
Enhancement of human cord blood CD34+ cell-derived NK cell cytotoxicity by dendritic cells.J Immunol. 2001 Feb 1;166(3):1590-600. doi: 10.4049/jimmunol.166.3.1590. J Immunol. 2001. PMID: 11160200
-
Immunomodulatory impact of α-fetoprotein.Trends Immunol. 2022 Jun;43(6):438-448. doi: 10.1016/j.it.2022.04.001. Epub 2022 May 9. Trends Immunol. 2022. PMID: 35550875 Review.
-
Natural killer cell activation by dendritic cells: balancing inhibitory and activating signals.Cell Mol Life Sci. 2011 Nov;68(21):3505-18. doi: 10.1007/s00018-011-0801-8. Epub 2011 Aug 23. Cell Mol Life Sci. 2011. PMID: 21861182 Free PMC article. Review.
Cited by
-
Tumor-derived α-fetoprotein impairs the differentiation and T cell stimulatory activity of human dendritic cells.J Immunol. 2014 Dec 1;193(11):5723-32. doi: 10.4049/jimmunol.1400725. Epub 2014 Oct 29. J Immunol. 2014. PMID: 25355916 Free PMC article.
-
Identification of Clinical Phenotypes and Related Survival in Patients with Large HCCs.Cancers (Basel). 2021 Feb 3;13(4):592. doi: 10.3390/cancers13040592. Cancers (Basel). 2021. PMID: 33546234 Free PMC article.
-
The role of natural killer cells in hepatocellular carcinoma development and treatment: A narrative review.Transl Oncol. 2019 Aug;12(8):1092-1107. doi: 10.1016/j.tranon.2019.04.021. Epub 2019 Jun 6. Transl Oncol. 2019. PMID: 31176993 Free PMC article. Review.
-
Non-secretion of AFP and neutrophil lymphocyte ratio as predictors for survival in hepatocellular carcinoma patients treated with sorafenib: a large UK cohort.Oncotarget. 2018 Mar 30;9(24):16988-16995. doi: 10.18632/oncotarget.24769. eCollection 2018 Mar 30. Oncotarget. 2018. PMID: 29682199 Free PMC article.
-
Route of antigen delivery impacts the immunostimulatory activity of dendritic cell-based vaccines for hepatocellular carcinoma.J Immunother Cancer. 2015 Jul 21;3:32. doi: 10.1186/s40425-015-0077-x. eCollection 2015. J Immunother Cancer. 2015. PMID: 26199728 Free PMC article.
References
-
- Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and trends. Gastroenterology. 2004;127:S35–50. - PubMed
-
- Bosch FX, Ribes J, Diaz M, Cleries R. Primary liver cancer: worldwide incidence and trends. Gastroenterology. 2004;127:S5–16. - PubMed
-
- Takayasu K, Arii S, Ikai I, et al. Liver Cancer Study Group of Japan Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–9. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Eng J Med. 2008;24:378–90. - PubMed
-
- Doherty DG, O'Farrelly C. Innate and adaptive lymphoid cells in human liver. Immunol Rev. 2000;174:5–20. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

